Ģąņåģąņč÷åńźą’
ģīšōīėīćč’.
Żėåźņšīķķūé
ģąņåģąņč÷åńźčé č ģåäčźī-įčīėīćč÷åńźčé ęóšķąė. - Ņ. 17. -
Āūļ. 3. -
2018. - URL:
http://www.sci.rostelecom67.ru/user/sgma/MMORPH/TITL.HTM
http://www.sci.rostelecom67.ru/user/sgma/MMORPH/N-59-html/TITL-59.htm
http://www.sci.rostelecom67.ru/user/sgma/MMORPH/N-59-html/cont.htm
ŌĆĮĪÓ ĀĪ
«Ńģīėåķńźčé ćīńóäąšńņāåķķūé ģåäčöčķńźčé óķčāåšńčņåņ»
Ģčķčńņåšńņāą
ēäšąāīīõšąķåķč’ Šīńńčč
Ļīä
šåäąźöčåé Ļóćą÷åāą Ģ. Ź.
Textbook of
HUMAN HISTOLOGY
Ńģīėåķńź 2018
Ļšåäčńėīāčå
Ó÷åįķīå ļīńīįčå īńķīāąķī
ķą ļåšåāīäå šóńńźīćī ņåźńņą ėåźöčé Ń. Ė.Źóēķåöīāą č Ģ. Ź. Ļóćą÷åāą «Ėåźöčč ļī
ćčńņīėīćčč, öčņīėīćčč č żģįščīėīćčč», ļšåņåšļåāųčõ 3 čēäąķč’. Ļīńėåäķåå āūųėī ā
ńāåņ ā
Ā źą÷åńņāå čėėžńņšąöčé
ņåźńņą čńļīėüēīāąķū ščńóķźč čē ó÷åįķčźą ļī ćčńņīėīćčč ļīä šåäąźöčåé Ž. Č.
Ąōąķąńüåāą č Ķ. Ą. Žščķīé, čēäąķķīćī ā 1999ć.
Ņåźńņīāūé ģąņåščąė č
ļīäščńóķī÷ķūå ļīäļčńč ļåšåāåäåķū ń šóńńźīćī ’ēūźą ķą ąķćėčéńźčé ąāņīšīģ
ķąńņī’łåćī ó÷åķīćī ļīńīįč’, ńīīņāņńņāóžłåćī ó÷åįķīé ļšćšąģģå ļī šąēäåėąģ
ćčńņīėīćčč č öčņīėīćčč.
Ä.ģ.ķ., ļšīōåńńīš Ļóćą÷åā Ģ.
Ź.
Šåöåķēåņķū
Ńņąšųčé ļšåļīäąāąņåėü źąōåäšū čķīńņšąķķūõ ’ēūźīā ŃĆĢÓ
Źīāąėüźīāą Ģ. Ā.
Ńņąšųčé ļšåļīäąāąņåėü źąōåäšū čķīńņšąķķūõ ’ēūźīā ŃĆĢÓ Čńąźīāą
Å. Ā.
Ēąāåäóžłą’ źąōåäšīé čķīńņšąķķūõ ’ēūźīā ŃĆĢÓ Ķčźīėąåāą Ņ. Ā.
CONTENTS
Introductory Remarks ..7
Chapter 1 Structure of cells ..8
Cell Study & Cytoplasm Structure .8
Endocytosis
13
Phagocytosis
..13
Contacts
between adjoining cells
15
Organelles of
ells
18
Chapter 2 Nucleus & The cell division ...31
The Cell
Cycle ..
36
The
Mitosis
.
39
The
Meiosis
.44
Capter 3 General Histology & Epithelial Tissues 51
Epithelial
Tissues
.
..54
The
Glands
.
.65
Chapter 4 Blood . ..70
Erythrocytes
..72
General
Features of Lucocytes
79
Blood
Platelets (Thrombocytes)
88
Chapter 5 Connective Tissue ..92
Loose
Collagen Connective Tissue
92
Dense
Collagen Connective Tissue
. 110
Connective
Tissue with Special Properties
..112
Chapter 6 Skeletal (Cartilage and Bone) Tissues . 115
Cartilage
Tissues
.
.115
The Bone
..122
Chapter 7The Muscle Tissue ..138
Smooth Muscle
Tissue
..138
Skeletal
Muscle Tissue
..142
Cardiac
Muscle
.
.
..151
Chapter 8 The Nervous Tissue ..154
Neuroglial
Cells
.
..159
Nerve
Fibers
.
164
Nerve
Endings
.
.169
Synapses
.
..176
Chapter 9 Nervous System (Spinal Cord & Peripheral Nerve & Sensory
Ganglia) .. 181
Peripheral
Nerves
..
..183
Sensory
Ganglia
..
.183
The Spinal Cord
187
Chapter 10 The Brain ..194
Cerebellum
.....197
The Cerebral
Cortex
201
The Autonomic
Nervous System
..
.211
Chapter 11 Sensory Organs & Eye and Olfactory Organ .. 215
Organ of
Vision
..215
The Olfactory
Organ Development and Function
.
.237
The
Vomeronasal Organ
.
238
Chapter 12 The Ear & Gustatory Organ 240
The External
Ear
.
240
The Middle
Ear
.
..241
The Internal
Ear
.
.242
The Cochlear
Labyrinth
.
245
The
Vestibular Membranous Labyrinth
.
.252
Gustatory
Organ
.
.257
Chapter 13 Cardiovascular Systems . ...261
Blood
Vessels
.
..261
Microcirculatory
bed
.
..266
Capillary
Vessels
.
268
Venules
272
Arteriovenous
Anastomoses
273
Veins
...276
Chapter 14 Lymphatic Vessels & Heart . 280
The
Heart
.
286
Chapter 15 Endocrine System Central Organs . .297
The
Hypothalamus
.
.299
The
Hypophysis
.
.304
The Pineal
Gland (Epiphysis)
.
311
Chapter 16 The Peripheral Endocrine System . .315
The Thyroid
Gland .
..
.315
The
Parathyroid Glands
..
..322
The
Suprarenal Glands (Adrenal Glands)
..
..324
Diffuse Endocrine
System
...
.331
Chapter 17 Hemopoietic Organs & Bone Marrow & Hemopoiesis 333
Hemopoiesis
in the Bone Marrow
...
.334
Blood
Formation in the Bone Marrow
..338
The Hemopoietic
Cell Classes
.. 338
Development
of the Neutrophilic Granulocytes to the Myeloblast Stage 239
The
Erythrocytopoiesis
..342
Chapter 18 Lymphatic Organs & Lymphocytopoiesis .348
The
Thymus
348
Lymphatic
Nodes
..353
The Spleen
..
.361
Palatine
Tonsils
...
368
Mucosa
Associated Lymphoid Tissue in the Alimentary System
371
Chapter 19 Anterior Part of the Alimentary Apparatus 373
The
Alimentary System Anterior Part
..377
Large
Salivary Glands
..386
Chapter 20 Teeth & Oesophagus . .394
Teeth
.394
The
Oesophagus
.
...408
Chapter 21 The Stomach & Small Intestine . .. ... 413
The Stomach
..
..... 413
The Small
Intestine
..
. 422
Chapter 22 Middle and Posterior Parts of the Alimentary
Canal . 432
The Large Intestine
.
432
The
Liver
.
...436
The
Pancreas
.
..449
Chapter 23 Respiratory Apparatus .. . 456
The Nasal
Cavities
.
456
The
Pharynx
..
.458
The
Larynx
..
459
The
Trachea
..
..460
The
Lungs
464
The Lung
Respiratory Part
..467
Chapter 24 Skin and its Appendages .. 477
The
Epidermis
..
..477
The
Dermis
..
...483
The Sweet
Glands
..
.486
The Sebaceous
Glands
490
Hairs
...
490
The
Nail
..493
Chapter 25 Urinary Organs . 495
The
Kidneys
495
The Kidney
Endocrine System
...507
The Urine
Passages
.512
Chapter 26 Male Reproductive organs . ..515
The
Testis
515
The
Spermatogenesis
..519
The Seminiferous
Tracts
.525
The
Prostate
527
The
Penis
531
Chapter 27 Female Reproductive Organs .533
The Ovary
Structure
..
533
The
Ovogenesis
..
538
The
Ovulation
..
..539
The Corpus
Luteum
..
.540
Uterine
Tubes
..
..544
The
Uterus
..
544
The
Vagina
..
...547
The Sexual
Cycle
..
. 548
The
Vulva
.
..552
Mammary
Glands
.
..554
INTRODUCTORY REMARKS
Histology is the study of cells,
tissues, and organs as seen by a microscope. This science includes general
histology and proper histology. General histology investigates development,
structure, and function of tissues. Proper histology investigates development,
structure, and function of every tissue and organ.
Cytology includes general cytology
and proper that. General cytology studies development, structure, and function
of cells. Proper cytology studies development, structure, and functions of
every organ cells.
Respected reader, the textbook of
human histology introduces You with:
1. Development, structure, and
function of cells.
2. Development, structure, and
function of every organ cells.
3. Development, structure, and
function of tissues.
4. Development, structure, and
function of symplast.
5. Differentiation of cells, tissues,
and organs.
6. Regeneration of cells, tissues,
and organs.
7. Nervous system influences the
development, structure, and function of cells, tissues, and organs.
8. Endocrine system influences the
development, structure, and function cells.
9. Adaptation of cells, tissues, and
organs to surroundings.
10. Alteration of cells, tissues, and
organs depend on age.
11. Surroundings influence the
development, structure, and function of cells, tissues, and organs.
CHAPTER 1
STRUCTURE
OF CELLS
The cell was revealed by Guck in 1665. Scientists Purkinje, Brown, Swann and Wirchov made an important contribution to the cell theory. In 1830 Purkinje discovered the cell cytoplasm. In 1833 science worker Brown discovered the cell nucleus. In 1838 scientist Swann came to drew conclusion, that all cells (animals and plants) were identical. In 1858 science worker Wirchov discovered, that new cells created by the division of maternal cells.
Cell Theory
1. The cell is the smallest unit of an alive organism.
2. All cells of different organisms have identical structure.
3. New cells are created by the division of maternal cells.
4. Multi cellular organisms contain many cells. The cells unify with each other and form tissues and organs being regulated by nervous system, by endocrine and immune systems.
The myofiber is the protoplasmic cord (or belt), which includes many
nuclei. It is called simplast.The net of cells is the group of cells connected
with each other by the cytoplasm bridges. It is called syncytium.
The cell is the primitive alive system, which includes the nucleus and the cytoplasm and serves as the basis of the development, structure and function of the organism.
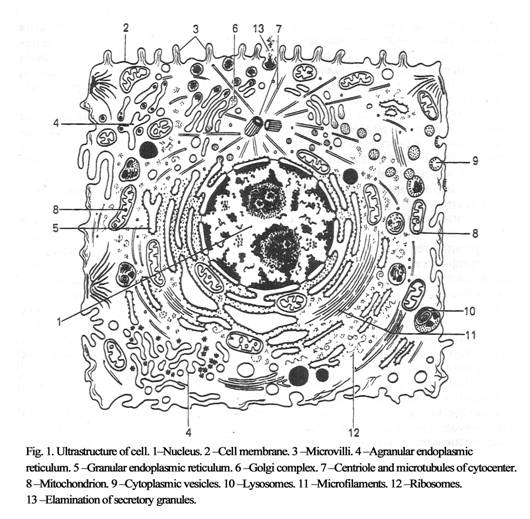
Cell study &Cytoplasm
structure
The cytoplasm includes the
hyaloplasm and organelles (Fig. 1).
The fluid hyaloplasm is
called cytosol, and the dense hyaloplasm is called gel. The hyaloplasm is the
solution, which includes minerals, carbohydrates, amino acids and enzymes. The
concentration of K+ is greater within the cell and less outside the
cell. The cytoplasm of a cell includes NaCl (0.9%). If the cell is put into
distilled water, the cell will swell. If the cell is put into hypertonic
solution or concentrated solution of glucose the cell will decrease in size.
Hyaloplasm functions
Anaerobic oxidation, self-formation of microtubules and microfilaments occurs in hyaloplasm. Subunits of ribosome and RNA pass through the hyaloplasm.
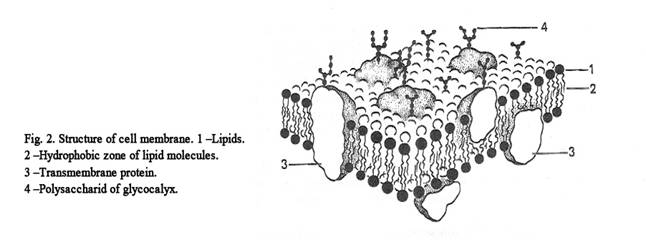
Membranes
They are represented by cell membranes and intracellular
membranes. Membranes are made up of lipids and proteins (Fig. 2).
All membranes have electoral permeability.
Intracellular membranes include cholesterol, phospholipids and sphingomyelins. Lipid molecules form a bi-layer. Every molecule has the hydrophilic head and the hydrophobic tails. The hydrophobic tails are in contact with each other; but the hydrophilic heads are not. The properties of lipid molecules are as follows: lipids can assemble with each other, recover themself and possess fluidity. The protein molecules include aminoacids. The part of a protein molecule, which includes amino acids with the charge are directed to the heads of lipid molecules. The part of a protein molecule, which includes amino acids without the charge, is directed to the tails of lipid molecules.
Membrane proteins are classified according on the arrangement in three groups: the integral, semi-integral and overlie-membrane proteins. Integral proteins are plunged into two lipid layers of membrane, semi-integral proteins are plunged into one lipid layer, overlie-membrane proteins arrange on the surface of lipid layers.
The properties of proteins molecules of the membranes are as follows: protein molecules have the ability to move alon a membranes, have to revolve on its axis, also axial rotation change.
Membrane proteins are classified according to their function. 1. Transitional proteins. 2. Enzymatic proteins. 3. Structural proteins. 4. Receptive proteins. The thickness of the intracellular membrane is 6 nm.
The thickness of the cell membrane is 10 nm. The thickness of the cell membrane is greater than that of the intracellular membrane because the cell membrane includes glycocalyx. The latter is made up of glycolipids and glycoproteins. The thickness of the glycocalyx is about 4 nm. Under the cell membrane the cortical layer lies. The layer includes retraction proteins (actin, myosin, tropomyosin, alpha-actinin). The functions of cell membrane are as follows. 1. Transport function. 2. Barrier function (the cell membrane separates the cytoplasm of a cell from the environment). 3. Receptor function.
Transport function
Small molecules, macromolecules and fluid droplets pass through the cell membrane. The small molecules (ions, water molecules, amino acids) may be transported under the influence of concentration of gradient and against the gradient. If molecules transport against the gradient of concentration, the energy is needed. The energy is produced by the ATP. There are special ATP-enzymes for transport N+ and K+. If molecules pass owing to the spent energy it is active transport. When the molecules transport under the influence of concentration gradient, the energy is not needed it is the passive transport.
Receptive function
Receptors include glycolipids and glycoproteins. Receptors may be scattered on the cell membrane surface or may be concentrated in some area. The cells recognize each other owing to the receptors. Owing to the receptors the cells unite with each other and form tissues and organs. The receptors can hold hormones, antigens, antibodies and erythrocytes of ram. When the receptors receive hormone then an adenylate cyclase is synthesized. The adenilate cyclase is called the signal molecule. The signal molecule stimulates the formation of the cyclic adenosine monophosphate
(cAMP). The cAMP activates the enzymes of the cell. Large molecules (macromolecules) and particles can enter cells by endocytosis.
Endocytosis
An endocytosis is classified in a phagocytosis and a pinocytosis.
Phagocytosis
The cells use the process of
endocytosis to engulf foreign matter (e.g. bacteria) and large molecules, and
particles. The process is then referred to as phagocytosis. Phagocytosis
includes three phases: adhesion, invagination and separation. First the
particle conjugates to the cell membrane, then the cell membrane invaginates
itself into the cell cytoplasm, then the invaginating part of a cell membrane
separates from the rest of the cell membrane (Fig. 3) to form a phagosome. (The
particle surrounded by the membrane is called phagosome).
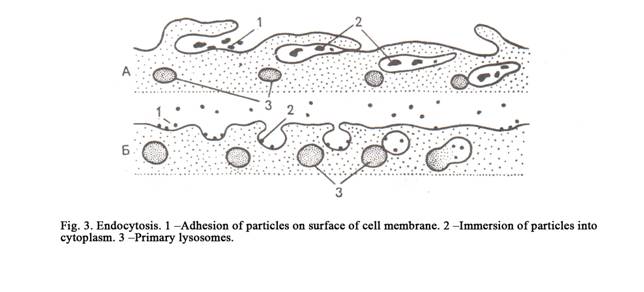
We can see that the square of cell membrane is vanishing in phagocytosis, that area of the cell membrane is decreased.
Pinocytosis
It is similar to phagocytosis, but droplet of fluid enters the cell. The fluid surrounded by the membrane is called pinocytotic vesicle.
Exocytosis
Small structures of cell cytoplasm (secretory granules, residual bodies) may be transported to the outside. This process is called exocytosis. Secret granules, residual bodies and other small structures are surrounded by the membrane. Thes structures approach to a cell membrane and fuse to the internal surface of a cell membrane. The vesicle membrane (secretory granule) and cell membrane undergo ruptures and secrete releases to the exterior. The vesicle membrane forms a part of the cell membrane therefore the area of the cell membrane increases.
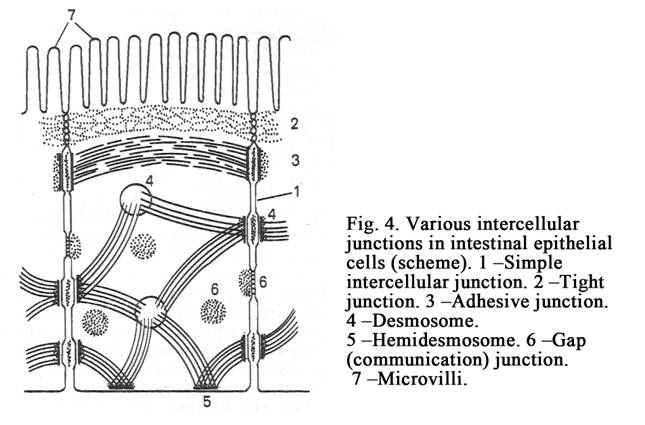
Contacts between Adjoining Cells
Tissues consisting of cells do not fall into single pieces
because
between cells there are adhesive proteins (cell adhesion molecules
CAMs) and intercellular contacts (cell junctions between cells). There are simple intercellular junctions, tight junctions (belt desmosomes), desmosomes (spot of punctum), gap junctions (nexuses), digiform junctions (Fig. 4), and synapses between neurons.
Simple intercellular junctions
The distance between cell membranes of adjoining cells may be about 20 nm. Special anchoring structures (cell adhesion molecules, special filaments) between such adjoining cells are absent. The space between cell membranes of the adjoining cells is loose. Such contacts between cell membranes of the adjoining cells are called simple intercellular junctions (Fig. 4. 1). Such contacts are observed between cells of connective tissue.
Tight Junctions (zonula occludens)
In this junction cell membranes of the adjoining cells connect tight with each other (Fig. 4. 2). The tight junction is similar to a belt. The belt surrounds the epical part of the epithelial cell and closes the intercellular space. Such contacts are observed between cells of epithelial tissues.
Intermediate junctions (belt, desmosomes, zonula adherens) The junctions include 2 thin belts, which surround the apical part of the cell.
There are integral adhesive
glycoproteins between the belts of the adjoining cells. The integral adhesive
glycoproteins connect cells with each other. Proteins and superfine fibrils are
attached to the cytoplasm aspects of cell membranes of the adjoining cells.
Intermediate janction are present 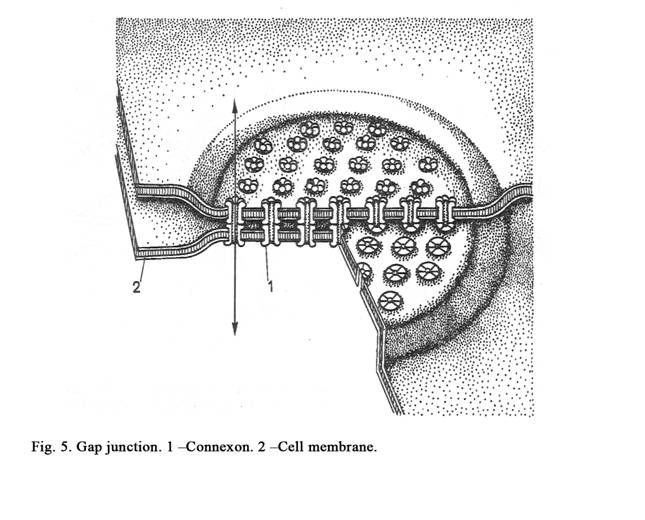
between cells of epithelial tissues.
Desmosome (spot of punctum)
A desmosome (Fig. 4. 4) is small round area of attachment (0.5 micrometer in diameter). At the site of a desmosome the cell membrane (of each cell) is thickened. There is a layer-like structure between thickening cell membranes of adjoining cells. There are dense substance and thin fibrils at the cytoplasm aspects at the site of the desmosome. Desmosomes are present where strong anchorage between cells is needed e.g. between epithelial cells of the epidermis.
Gap junction (nexus)
In these junctions plasma membranes do not come in actual contact (as in tight junctions), but lie very close to each other, the gap being reduced to 2-3 nm. The diameter of the gap junction area of is about 1000 nm. A minute canaliculus passing through the gap junction connects the cytoplasm of the two cells (Fig.4. 5 & Fig.5) thus allowing free passage of some substances (sodium, potassium, calcium, the water molecules). Gap junctions are present between smooth muscle cells and cardiac myocytes.
Digiform intercellular junctions
At the site of the junction the plasma membrane of one adjoining cell invaginates into other adjoining cell. These contacts provide mechanical connection between adjoining cells. There are these contacts between cells of epithelial tissues.
The synapse
The synapse connects nervous cells or its processes with each other. Nervous impulses pass through the synapse from one nervous cell into other cell (from presynaptic membrane to postsynaptic membrane).
Organelles of Cells
The organelles are constant cell structures performing special functions. Organelles are divided in 1) organelles, which consist of
membranes (membrane organelles), and non-membrane organelles, 2) constant organelles, and 3) special organelles.
Membrane organelles
These organelles include rough endoplasmic reticulum, smooth endoplasmic reticulum, the Golgi region, lysosomes, peroxysomes and mitochondria.
The rough endoplasmic reticulum (ER) contains a system of membranes. The membranes form flat sacs (or cisterns), tubules and vesicles (Fig. 6). The membrane external surface of these structures is covered by ribosomes. The rough endoplasmic reticulum performs two functions 1) synthesis of protein and 2) transport of produced substances to the Golgi region. There are parallel rough membranes in the cytoplasm.
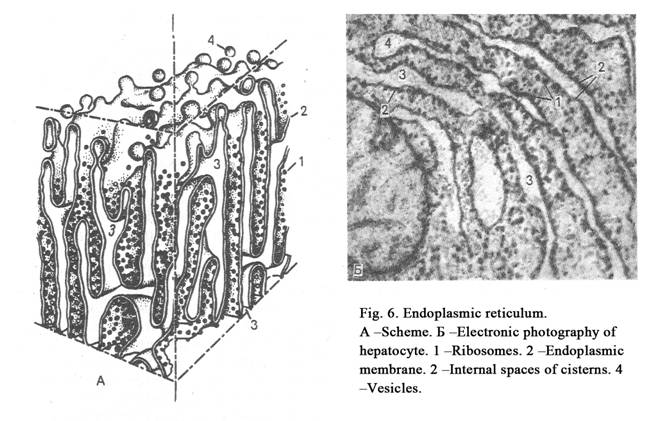
If the rough endoplasmic reticulum is developed into the cell well, the cell is mature and releases protein for the export.mooth endoplasmic reticulum includes tubules, cisterns and vesicles surrounded by membranes without ribosomes. The smooth ER performs functions as follows: 1) Synthesis of carbohydrates, lipids and steroid hormones. 2) Destroy of poisons. 3) Accumulation of ions Ca++ into cisterns. 4) Transport of produced substances to the Golgi region.
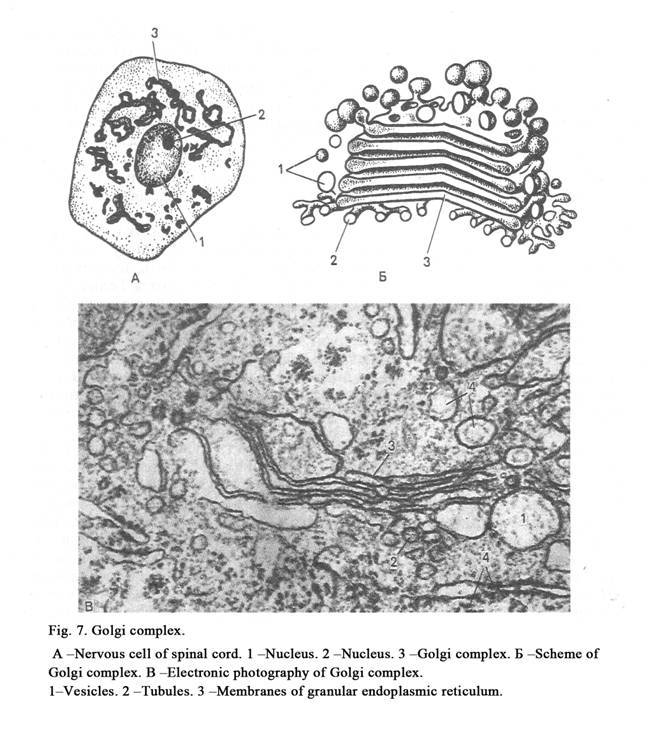
The Golgi complex (Golgi region) is made
up of a number of flat cisterns surrounded by smooth membrane. These cisterns
are stacked over one another (Fig. 7). Some cisterns form the cis-face, medial
Golgi, and trans-face. They connect with each other by vesicles and tubules.
The Golgi region is located over the nucleus into glandular cells, surrounds
the nucleus
into nervous cells, forms a cap for nucleus in chromaffin cells of adrenal
medulla, and is scattered in some cells.
Functional significance of the Golgi region is as follows: 1) the Golgi region separates secretory productions from cytoplasm and forms vesicles; if the vesicles contain secretion, they are called secretory granules; if the vesicles contain enzymes, they are called lysosomes; 2) secretion is released from cell owing to the Golgi region; 3) the cell membrane recovers itself by the Golgi region (a membrane of granule forms a part of the cell membrane); 4) carbohydrates and other substances join with one other to form the secretory granules into the Golgi region; 5) the Golgi region takes part in the lysosomes formation.
Lysosomes are small (Fig. 8) vesicles surrounded by membrane. They contain enzymes. Acid phosphatase is marking enzyme of lysosomes. Classification of lysosomes is as follows. 1. Primary lysosomes. 2. Secondary lysosomes. 3. Tertiary lysosomes (residual bodies). Rough ER and the Golgi region form primary lysosomes. The diameter of a lysosome is about 200-400 nm. Secondary lysosomes are formed by fuse of primary lysosomes with phagosomes (foreign particles engulfed by the cell).
Foreign particles are destroyed inside the secondary lysosomes. Amino acids and other substances are formed inside the lysosome. They pass from the lysosome through the lysosomal membrane into the cytoplasm of a cell.
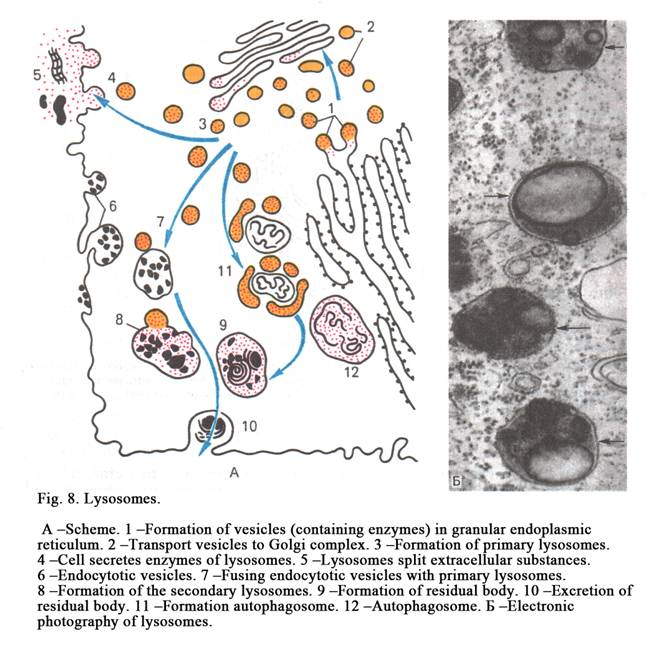
If primary lysosome unites with damaged organelle (with ribosome, mitochondria) it is called autophagosome. If cell includes many autophagosomes, it is indication that the cell is damaged. This may be by metabolic stress or by damage of the cell.
Tertiary lysosomes (residual bodies) are formed after the intercellular digestion ends. The containment of lysosome is not entirely destroyed by lysosomal enzymes. This containment is called residual body. Residual bodies move off by exocytosis.
The functions of lysosomes are as follows. 1. Participation in intracellular digestion. If the cell includes a number of lysosomes, it performs the phagocyte function. 2. Lysosomes protect cells from death. If the cell contains a few lysosomes, the cell will be destroyed therefore carbohydrates and lipids are accumulated.
Peroxisomes are similar to lysosomes in that they are membrane bound vesicles containing enzymes (it is 0.3 1.5 micrometer in diameter). Peroxisomes include two enzymes: peroxidasa and catalasa. Lysososomal enzymes oxidize amino acids therefore hydrogen peroxide is formed. Hydrogen peroxide is toxic for the cell therefore the hydrogen peroxide is destroyed by the enzyme peroxydasa. Catalasa is marking enzyme of peroxisomes.
Mitochondria have round-like or elongated shape. Mitochondria are vary in size, most of them being 0.5 to 2 micrometer in length. A mitochondria is bound by the smooth outer membrane within which is an inner membrane, the two bein separated by an inermembranous spce. The inner membrane is highly on itself forming incomplete partitions called criste. The space between criste is called the matrix. There are thin fibrils (2 to 3 nm in diameter) inside the matrix. The fibrils are metachondrion DNA.Also small granules (15 to 20 nm in diameter) are present; the granules are mitochondrion ribosomes (Fig. 9).
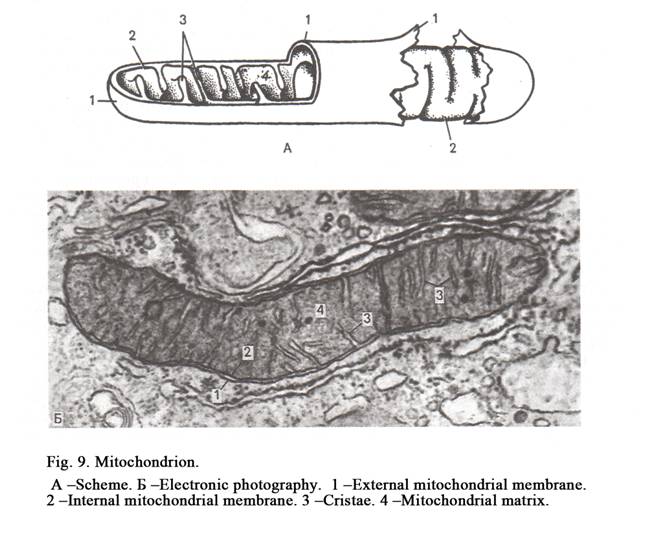
The functions of mitochondria are as follows. 1. Thirteen types of proteins are synthesized inside mitochondria. 2. ATP is synthesized inside phosphorylation).
Non-membrane organelles
They include ribosomes, cytocenter and microtubules.
Ribosomes are formed inside the
nucleolus of the nucleus. A ribosome includes two subunits one of which is
larger then the other. The size of the ribosome is 25x20x20 nm. The ribosome
contains ribosomal RNA and ribosomal proteins.
Ribosomes play an essentuil role in protein synthesis.
Ribosomes may be present singly, in this case they are called monosomes; they may form groups, which are referred to as polyribosomes (polysomes). Most of ribosomes are present in relation to rough ER. If
rough ER of the cell is developed well, the cell is mature and synthesizes protein for export. If rough ER of the cell is developed worse and includes many monosomes and polyribosomes, the cell is not mature and synthesizes protein for intracellular use.
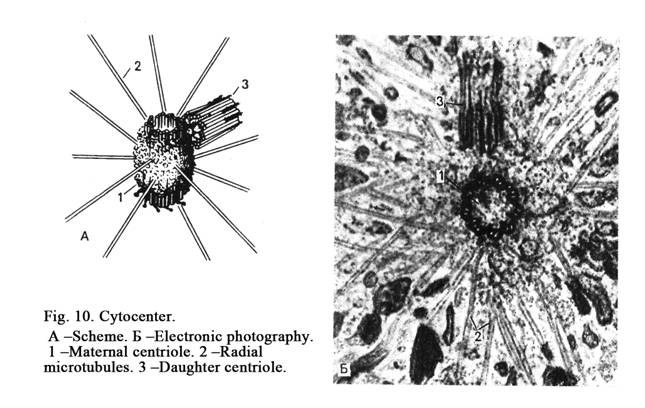
The cytocenter includes two centrioles (Fig. 10). One of which is called the maternal centriole, the other is called the daughter centriole. The daughter centriole lies perpendicular to the maternal centriole.
The centrioles have cylinder shape that is 200 nm in diameter and 500 nm in length. The centriole (cylinder) wall includes 9 triplets. Each triplet includes three microtubules.
Satellites (short outgrowths) run from microtubules. Many microtubules run from diplosome (cytocenter) in different directions. It is called centrosphera.
Centrioles of the cytocenter move either to the pole of the cell before cell division. In this case each centriole becomes maternal centriole. A new daughter centriole forms with the help of a maternal centriole. A new daughter centriole development is induced by a maternal centriole. Thus there are two cytocentres inside the cell before division.
The cytocenter functions are as follows. 1. The cytoskeleton microtubules are formed by the maternal centriole during the interphase. 2. The maternal centriole induces formation of a new daughter centriole in the end of the interphase. 3. The mitotic spindle is formed by cytocenter during the mitosis.
Cytoskeleton includes microtubules, microfilaments, and ntermediate filaments.
Microtubules form a part of mitotic spindle of cell division. In the interphase cell microtubules form the cytoskeleton, are included in flagellum, cilium, and centriole. A microtubule external diameter is about 24 nm, internal diameter -15 nm, the wall thickness -5 nm. Microtubules include protein tubulin. The tubulin form rings. The rings are stacked over one another to form protofilaments. Every microtubule includes 13 protofilaments. A microtubule self-formation is induced by the maternal centriole inside the hyaloplasm. If the cell temperature is subnormal, a microtubule self-formation is stops, all other microtubules are destroyed, and the shape of the cell becomes irregular. Microtubules are destroyed by the influence of colchicines.
Functions of microtubules are as follows. 1. Microtubules form the cytoskeleton of the cell and keep the cell shape. 2. Microtubules take part in movement of cilia and flagella. There is a special protein (kinesin) inside the cell. The protein moves vesicles, mitochondria and other substances along microtubules in the cell cytoplasm.
Microfilaments or thread-like structures consist of retractive proteins (actin, myosin, tropomyosin, a-actinin). A microfilament is usually 6 nm in diameter. Microfilaments lie deep to a cell membrane and form sub-membrane layer (cortical layer of cell). If microfilaments of sub-membrane layer contract, a cell membrane is invaginated by phagocytosis, pinocytosis, and cell division during cytokinesis. Microfilaments take part in pseudopodia formation during cell movement.
The functional significance of microfilament is as follows: 1. Micro-filaments form cytoskeleton. 2. Microfilaments take part in intracellular movement of mitochondria, ribosomes, and vesicles, also take part in invagination of cell membrane by phagocytosis, pinocytosis and cytokinesis. 3. Microfilaments take part in extra cellular movement of the cell.
Intermediate filaments consist of special proteins. Intermediate filaments have rod-like shape. These filaments are 10 nm in diameter. Different tissue cells have different special proteins. Intermediate filaments of epithelial cells include protein keratin. Intermediate filaments of connective tissue cells include protein vimentin and intermediate filaments of smooth muscle cell include protein desmin.
Functional significance of intermediate filaments is as follows: 1) Are included into the cytoskeleton. 2) Tumor of tissues may be recognized with the help of intermediate proteins. If a tumor includes keratin, the tumor is developed from epithelial tissue etc.
Cilia are special moving organelles. Cilia are minute hair-like projections from the free surfaces of some epithelial cells. Each cilium is 5-10 micrometer in length and 300 nm in diameter. The inner core of a cilium includes an axoneme. The axoneme includes nine peripheral pairs of microtubules and one pair of central microtubules (2x9+2). The axoneme is connected to the basic little body. The axoneme is surrounded by cell membrane.
The functional significance of cilia is as follows. Cilia can perform ascilatory movement, round movement, hook-like movement. Owing to the movement of cilia foreign particles (bacteria, dust, mucous) are disposed from respiratory ways. But if a person smoking, cilia stop moving. In this case dust, bacteria and mucus are stored as at result bronchitis begins.
Flagella are projections of special cells. Each flagellum is 75-150 micrometer in length. The core of a flagellum includes the axoneme. The axoneme is surrounded by the cell membrane. A flagellum axoneme and a cilium are 200 nm in diameter. There is a flagellum in a spermatozoon.
A flagellum functions so that a spermatozoon can move in the fluid medium to.
Microvilli are projections on the apical part of some epithelial cells (the intestines, kidneys). The microvilli are 100 nm in diameter and 1000 nm in length. The microvili are surrounded by the cell membrane. There are fascicules of filaments in microvilli. The functions of microvilli are as follows. 1. Microvilli increase the cell surface. 2. Microvilli perform an absorption function in the intestines and kidneys.
Cytoplasm inclusions
These are non-constant components of cells, which appear and disappear in the cell and depend on the cell metabolism. Inclusions are divided into food (proteins, carbohydrates, fat), secretory vesicles, excretory granules (substances liable to releasing from the organism), and pigment. The latter is divided into external material (dust, dyes), and internal material (hemoglobin, myoglobin, lipofuscin, hemosiderin, melanin, lipochromes, bilirubine).
CHAPTER 2
NUCLEUS &
THE CELL DIVISION
The nucleus (Fig. 11) is usually rounded or ellipsoid. Occasionally it may be oval, indented or lobed. Sometimes the shape of the nucleus depends on the cell shape. For example, smooth muscle cell is spindle shaped, its nucleus is rod-like. The round or cubic cells have usually round nuclei. For example, nuclei of the blood lymphocytes are round in shape. Often the shape of the nuclei does not depend on the shape of the cells. For example, the blood granulocyte, having round shape, contains lobed nucleus. Nuclei of neutrophils (blood cells of women) contain nucleolar satellite (sex chromatin) resembling the appearance of a drumstick.
What is the nucleus? The nucleus is a
system of genetic determination and regulation of protein synthesis. What is
determination? It is a program of cell development. Thus, the nucleus performs
two functions: 1) keeps and transmits genetic material to daughter cells, 2)
regulates protein synthesis.
What is the first function performed?
Keeping the hereditary material is provided by reparative enzymes, which are
presented in DNA of chromosomes, and repair themselves after an injury. What
the hereditary material is transmitted to daughter cells? During the interphase
a molecule of DNA is replicated to form new resembling molecules of DNA. When a
maternal cell divides every daughter cell receives identical molecules of DNA
of chromosomes. What nucleus regulates protein synthesis? This process is
regulated by the transcription of the messenger RNA (m-RNA), ribosomal RNA
(r-RNA) and transfer RNA (t-RNA) from the surface of DNA of chromosomes. RNA
plays the vital role in the synthesis of proteins from amino acids. If the
quantity of RNA increases, protein synthesis occurs rather in the cell
cytoplasm. If the amount of RNA is less in the cytoplasm of the cell, the level
of the protein is lower. This way the nucleus regulates the synthesis of
proteins.
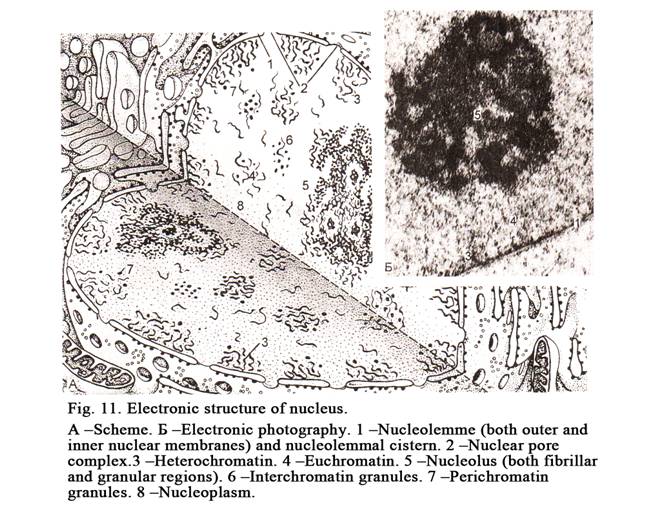
Nucleus Structure
The nucleus includes a chromatin,
nucleoli, a nuclear membrane, and nucleoplasm (Fig. 11). The interphase
chromatin is called so because it is able to stayned by basic dyes. What is chromatin?
These are recoiled chromosomes, which lose its regular shape. If DNA of
chromosomes is the most recoiled, chromatin becomes loose and is called the
euchromatin. If DNA of chromosomes is less recoiled, chromatin is condensed and
is called heterochromatin. The latter is not active, but euchromatin is active.
Why is heterochromatin inactive, but euchromatin active?
Heterochromatin is not active because the genes of DNA of the chromosomes are
closed, while the genes of DNA of chromosomes of the euchromatin are opened,
therefore the transcription of RNA from these genes is greatest; therefore the
quantity of RNA increases. This manner the synthesis of proteins is regulated
by the nucleus.
Both mitotic and interphase chromatin
contain fibrils which include 1 part of DNA, 1.3 part of histone and
non-histone proteins, and 0.2 part of RNA. The length of fibrils may be some
hundreds mm to
One can see thread-like structures
within chromatin. It is RNA molecules, which are transcribed from DNA of
chromosomes and called fibril rounding chromatin. These can coil to form
granules. Granules can join with proteins forming informative bodies. They may
by present within or round of chromatin.
Nucleoli
A nucleus contains 1- 3 nucleoli. They are formed on the surface of nuclear organizing centers of chromosomes. If the organizing centers of chromosomes are collected in one point of the nucleus, the latter contains one nucleolus only. If the organizing centers of chromosomes are located in any points of the nucleus, the latter contains more than one nucleolus. In the region of nuclear organizing centers of chromosomes there are some hundreds genes. Here ribosomal RNA (r RNA) is transcribed from genes of the chromosome DNA. After it r RNA joins with ribosomal proteins to form subunits of a ribosome.
The nucleolus consists of 2 components: 1) fibrillar part, localized in the center of the nucleolus, called the fibrillar region, 2) granular part, localized on the peripheral surface of the nucleolus, called the granular region. What is the fibrillarar region? It is r RNA transcribed from genes of the nuclear organizing center of chromosomes (the templates for the transcription). What is the granular region? It is ribosomal subunits. These are formed by r RNA joining with ribosomal proteins synthesized by rough ER of the cytoplasm and passing into the nucleus through nucleolar pores of the nucleolar membrane. There are large and small subunits that leave the nucleus through nuclear pores and are joined with one other to form the ribosome. They may connect with the membrane of rough ER and may lie free in the cytoplasm. They are called monosomes. Aggregations of ribosomes are called polyribosomes. Ribosomes are structures where proteins are synthesized. The more ribosomes are present in the cytoplasm of a cell, the more proteins are synthesized in it. Thus, nucleoli regulate the synthesis of proteins. The larger the nucleolus size, the more active the cell.
The nucleoli may disappear in normal and pathological conditions. When do nucleoli disappear in normal conditions? They disappear during mitotic division. While DNA of chromosomes begins coiling, its genes (the genes of the nucleolar organizing centers coil too) are closed as a result the transcription of r RNA is stopped. The formation of ribosomes cannot occur without r RNA during this period. Therefore the nucleoli disappear. The nucleoli may disappear after the cell have affected by toxic substances. Before disappearance of the nucleolus its granular region is separated from the fibrillar region, then both granular and fibrillar components or r RNA disappear.
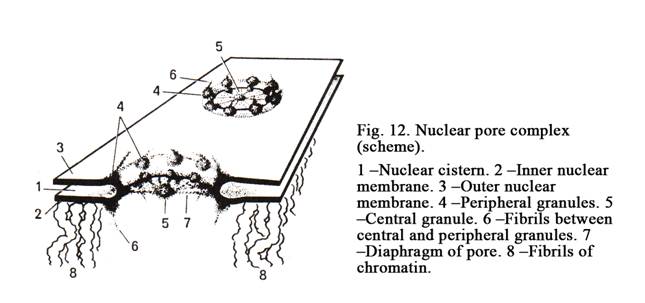
The Nuclear Membrane
It consists of the outer and inner membranes (Fig. 12). The outer membrane is continuous with rough ER. The space between the inner and outer membranes is called nucleolemmal cistern. Inner membrane is connected with chromatin and fibrillar nuclear component. The nuclear membrane has pores. They contain pore complexes. The pore complex includes the annulus of a pore about 90 nm in diameter, pore granules, and pore diaphragm. At several points the inner and outer membranes fuse to form the gap called the annulus. Pore granules form 3 layers. Every layer is made up of 8 protein granules, which are about 25 nm in diameter, located in the periphery of the pore annulus. The external layer of granules found near the cell cytoplasm, the internal layer is directed towards the nucleoplasm, the third layer is localized between the outer and inner layers. Every peripheral granule is connected with the central granule by thin fibril to form the diaphragm of the pore. The nuclear pores represent sites at which substances can pass from the nucleus and vice versa. If the nuclear membrane contains a number of pores the nucleus is active.
The Cell Cycle
A period between two subsequent divisions is called the cell cycle. The period between the cell division and its death is the cell cycle too. The ell cycle includes 4 periods. The first period is called postmitotic interval (G1 phase), the second period is called genetic synthesis (S phase), the third period is called premitotic interval (G2 phase), and the cell division is called mitotic period (Fig. 13).
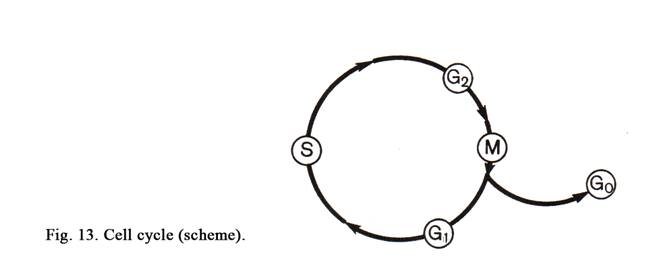
After mitotic division 2 daughter cells are formed and a new postmitotic interval advents. At the same time daughter cells are small, the quantity of the protein is little, amount of chromosomes and DNA is 2n and 2c respectively. During postmitotic interval on the DNA surface precursors of DNA, enzymes, and proteins are synthesized, as a result the daughter cells become large. At the same time basic processes are synthesis of proteins and receptors. The G1 phase may last from a few hours to many years. After the postmitotic interval S phase advents. During this period DNA molecules are replicated to form 4c and 4n (the nucleus contains 46 maternal chromosomes, each including 2 daughter chromosomes). During the G2 interval different RNA are copied off from the surface of DNA. This process is called transcription. At the same time special protein (tubulin) and ATP are synthesized. This is required for mitotic spindle formation and cell division. After the premitotic interval mitotic period begins.
Some cells can leave the cell cycle. The exit of the cell from the cell cycle is marked as the G0 phase. Such cells cant divide. They begin differentiating. Some cells temporarily losing the ability to divide can return into the cell cycle, but other cells undergo further differentiation and die. After initial differentiation the cell performs vary functions, then return into the G1 of the cell cycle, then enter the S phase and the G2 interval respectively, then begin dividing. What organs or tissues of the human body do the G0 cells contain? Such cells are present in the liver. But if the liver is injured its cells (hepatocytes) return in the cell cycle, begin dividing as a result this organ is fully restored. Stem cells are in a phase G0, but they can enter the cell cycle (G1, S and G2 phases) and are exposed to mitotic division. There are tissues and organs where the G0 cells undergo primary, then secondary differentiation, and die. After initial differentiation these cells perform a special function. Such cells are present in the blood, connective and epithelial tissues (for example epidermis). They may assume G1, S, G2 and G0 phases.
Tissues, which cells are often divided, injured more rapidly then tissues with slowly divided ones, because chemical and physical agents break down the mitotic spindle.
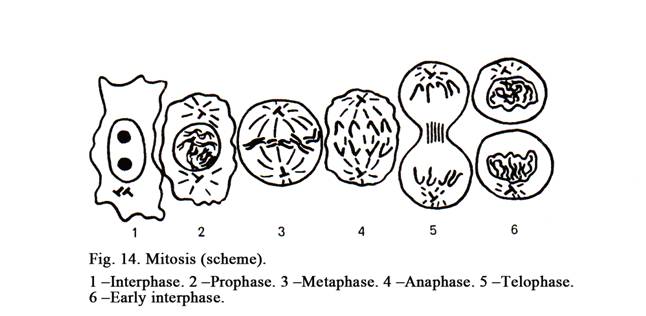
The Mitosis
The main distinction between mitosis and direct division is the transfer of identical genetic material to daughter cells, but during direct division the genetic material is not identical. Mitosis is divided into 4 stages called prophase, metaphase, anaphase, and telophase (Fig. 14).
If the cell nucleus contains half number of
chromosomes (23) it is called haploid number and is marked as 1n of chromosomes
and 1c of DNA. If the cell nucleus includes the complete number of chromosomes
(
Prophase
The prophase (Fig.14. 2) is divided into early prophase (compact chromatin) and late prophase (dispersed chromatin). At the early prophase chromosomes begin coiling therefore they are seen as thin threads to form a compact glob (compact chromatin). When the late prophase advents chromosomes go on coiling, the nuclear membrane breaks down and nucleoli disappear because genes of nucleolar organizing centers are closed resulting in the stoppage of the transcription of rRNA. It is known that the formation of the subunits of the ribosomes cant happen without rRNA. At the same time the nuclear membrane is fragmented. The fragments of the nuclear membrane are coiled to form vacuoles. Cisterns of ER are divided to form smaller structures. The number of ribosomes on the surface of rough ER is decreased therefore the synthesis of proteins is low. At the same time the cytocenter of the cell is divided to form 2 ones. These begin moving to opposite poles of the cell. Every new cytocenter is made up of 2 centrioles (maternal and daughter). Between them mitotic spindle is formed. The spindle is made up of microtubules. The chromosomes go on coiling to form a loss ball.
The Metaphase
At the metaphase (Fig.14. 3) one can see daughter chromosomes within maternal chromosomes. Maternal chromosomes move to a position midway between the two poles of the cell (at the equator of the cell). In the equator view chromosomes have an image a plate (the equatorial plate), in the pole of the cell view they have image of a star (the maternal star). At the metaphase the formation of the mitotic spindle is finished. There are 2 types of spindle microtubules. The first type of microtubules is formed by the cytocenter. These are called microtubules of the cytocenter. The second type of microtubules is formed by the centromere of chromosomes. They are called centromere microtubules. The ends of centromere microtubules are arranged between those of cytocenter microtubules.
The Anaphase
When anaphase (Fig.14. 4) advents daughter chromosomes (chromatid) simultaneously detach from each other and begin moving to the opposite poles of the cell to form two daughter stars (it is called diaster). Moving of daughter stars is provided by a mitotic spindle and moving of the poles from each other.
Mechanism of Daughter Stars Moving
This moving is provided by
chromosomal microtubules, which slide between telocentric microtubules to pull
chromatids of the daughter stars to the poles of the cell.
Telophase
After the end of the
anaphase (Fig.14. 6) moving of the daughter stars is stopped, and a nucleus
membrane is formed. Chromosomes undergo recoiling, the nuclear membrane
surrounds them, DNA genes become opened therefore RNA is synthesized. At the
same time DNA of chromosomes in the region of nucleolar organizing centers is
recoiled, their genes open, r RNA and subunits of ribosomes begin forming,
therefore nucleoli appear.
The nucleus division is
accompanied by the division of the cytoplasm. In this process the organelles
are duplicated and each daughter cell comes to have a full component of
them. At the same time cytokinesis
begins. At the equator of the maternal
cell its membrane is invaginated by microfilaments localized in this region,
resulting in the divided cell cleavage into separate cells. The mass of every
daughter cell is two times less then the mass of the maternal cell. The number
of chromosomes is 2n and the quantity of DNA is 2c in every daughter cell. The
mitotic division is the end of the cell cycle. The biological significance of
the cell division is important because it provides the growth of the body,
physiological and reparative regeneration of the cells, tissues and organs.
Pathological Mitosis & Cells
with Irregular Number of Chromosomes
Agents impairing cell
division are low temperature, chemical and physical factors, increased quantity
of cytocentres, chromosomal aberration. If low temperature, chemical substances
(colchicin) and physical agents influence the cell its spindle is destroyed and
cell division is stopped. If the dividing cell contains 3 or more cytocentres,
it is divided into 3 or more daughter cells, containing irregular number of
chromosomes.
Chromosomal Aberration
If affect the cell
ultra-violet or radioactive rays, arm of one or more of chromosomes may detach.
In this case one daughter cell receives some (45.5) chromosomes, all the
chromosomes are rounded by the nuclear membrane to form one large nucleus.
Other cell receives some normal chromosomes and the arm of the broken
chromosome (46+0.5). Both the arm of the broken chromosome and the rest
chromosomes are surrounded by the nuclear membrane to form 2 nuclei
(micronucleus and macronucleus).
In another case of the
aberration the arms of both daughter chromosomes may be adhered. By the
anaphase these chromatids lie longitudinally along the mitotic spindle and
connect both daughter stars. After the division of the maternal cell detached
arms may appear within the first or the second daughter cell. These contain
irregular number of chromosomes too.
Amitosis
This division is
characterized by the fact that the nucleus and the cytoplasm are divided by
constrictions as a result genetic material of the daughter cells may be not
equal.
The Polyploidy
It is increased number
of chromosomes as a result polyploidy nucleus is formed. The formation of polyploidy
cells occurs due to two mechanisms: 1) blocking either of a mitotic phase, 2)
interference of the maternal cells cytokinesis.
Demonstration of the First Mechanism
Lets take blocking G2 phase
as an example. In this case the cell contains 4n of chromosomes and 4c of DNA.
Then begins phase G1, and then the cell enters the S phase. At this phase DNA
is replicated. Thereafter the cell contains 8n of chromosomes and 8c of DNA.
Then the cell enters the G2 phase, after it dividing begins. After this process
every daughter cell contains 4n of chromosomes and 4c of DNA.
Demonstration of the Second
Mechanism
After the interference
of the cell cytokinesis the cell does not divide as a result this cell contains
2 nuclei. Every nucleus includes 2n and 2c. After it the cell enters G1 phase,
then S phase. At this phase DNA is replicated. Thereafter every nucleus of the
cell contains 4n and c. Then mitotic
phase begins. (The maternal star of the second nucleus). After division every
daughter cell contains 4n and 4c.
Duplication of DNA without Cytoplasm
Division
It is subsequent many
times repeated duplication of DNA without division of the cytoplasm, which
results in the increased of the chromosome number. These chromosomes are
connected with each other by thin threads. Such cells are located in the
placenta.
Meiosis
The cells (gametes)
formed by meiosis contain the haploid number of chromosomes (1n). We will study
spermatogenesis. It is divided into 4 periods: 1) multiplication; 2) growth
(prophase); 3) maturation is divided into 2 meiotic divisions (the first and
the second divisions), 4) formation of the spermatozoon (it will not be
studied).
Multiplication
The divided cells are
called spermatogonia. These cells lie near the basal lamina. The spermatogonia
undergo several mitotic divisions, finally containing 2n and 2c. Then they
enter the cell cycle (G1, S, and G2). Thereafter these cells contain 4n and 4c
(46 maternal and 92 daughter chromosomes). Then cells enter the growth period,
they are called primary spermatocytes.
Growth Period
The growth period is
divided into 5 phases: leptotene, zygotene, pachytene, diplotene, and
diakinesis.
Leptotene
Chromosomes recoil and resemble thin
threads.
Zygotene
At the same time
homologous chromosomes are joined with each other to become bivalent. There are
4 chromatids in every bivalent because every maternal chromosome includes 2
chromatids (1 central and 1 peripheral, i.e. the bivalent contains 2 central
and 2 peripheral chromatids). The two central chromatids (one belonging to each
maternal chromosome of bivalent) become coiled over each other. This process is
called crossing over. At the site where the chromatids cross they become
adherent: the points of the adhesion are called chiasmata. The crossing over
results in the exchange of genetic material between central chromatids. Every
chromatid of the bivalent now has distinctive genetic content and the bivalent
is usually called quadrivalent chromosome. The maternal chromosomes, included
in the quadrivalent chromosome, are now usually called double chromosomes
because they contain two chromatids, including distinctive genetic material;
therefore these chromatids are called monads. Primary spermatocytes now contain
23 quadrivalent chromosomes, 46 double chromosomes (diads) and 92 monads.
Pachytene
In this phase double
chromosomes and monads go on coiling to become shorter and thicker. The two
monads of each double chromosome become distinct because between monads one can
see splits.
Diplotene
Two double chromosomes
of a quadrivalent chromosome now try to move apart. Their central monads are
joined at the point of crossing over therefore they cannot move apart.
Diakinesis
By diakinesis the monads
go on coiling as a result they become shorter and thicker. At the same time
every primary spermatocyte contains 23 quadrivalent chromosomes, 46 doublet
chromosomes, and 92 monads.
Maturation Period & First
Meiotic Division
During the first division 23 quadrivalent chromosomes are placed at the
equator. Then double chromosomes move to the opposite pole of the spermatocyte.
After division of the primary spermatocyte 2 secondary spermatocytes are
formed. Every secondary spermatocyte contains 23 double chromosomes and 46
monads.
The second meiotic division
The double chromosomes
are placed at the equator, ater it monads move to each pole of the spindle.
After division of the secondary spermatocyte two spermatids are formed. Every
spermatid contains 23 monads or chromosomes (1n).
The Structure of Mitotic Chromosomes
Mitotic chromosomes appear during
the mitosis. They are seen in the metaphase (maternal aster) and anaphase
(diaster). In the aster one can see that every maternal chromosome consists of two
daughter chromosomes or chromatids. Every chromosome contains one molecule
(fine fibril of chromonema) of DNA, which is packed up by particular manner and
assumes characteristic shape (Fig. 15). Every chromosome has primary
constriction where the centromere is located. The part of the chromosome
localized between the centromere and the chromosomal end is called the arm. If
the primary constriction is roughly localized midway between two ends of the
chromosome it is called metacentric chromosome. If one arm of the chromosome is
significantly longer then the other, this is referred to as submetacentric
chromosome. The ends of chromosome arms are called telomeres. Some chromosomes
have the second constrictions. These are nucleolar chromosome. The part of the
chromosomal arm between the secondary constriction and telomere is called the
satellite. The set of the chromosomes of the nucleus is called the karyotype.
The karyotype is number, size and peculiarity of structure of the chromosomes.
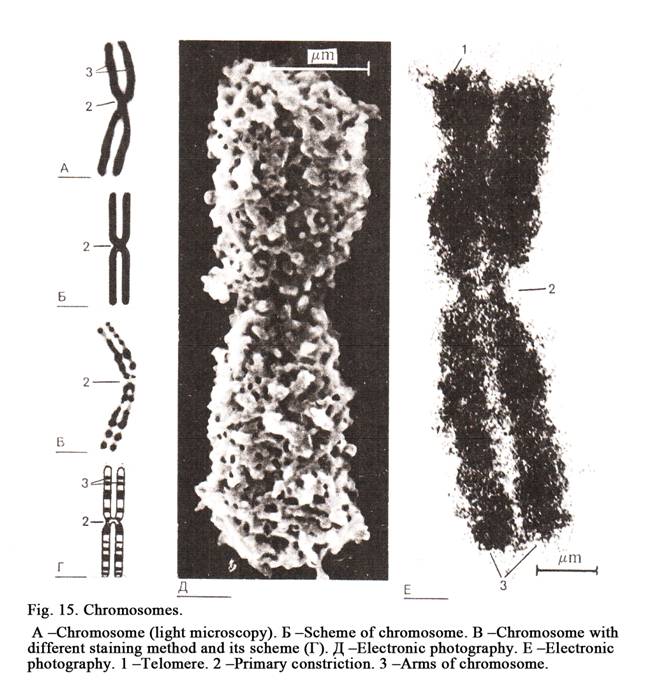
The chromosomes of the
human nucleus are divided into 7 groups and marked by the English Roman
alphabet capital letters from A to G. The structure of chromosomes of one group
is identical, but chromosomes the other groups are distinguished. In order to
distinguish the chromosomes within the group they must be stained by a special
dye (differential staining method) as a result the chromosomes show dark and
light bands. The pattern of bandings is specific for each chromosome.
Cell Reaction to External Influence
If the cell is under the
influence of chemical, physical or biological agents, it shows various
structural and functional disorders. The cell may either adapt to the new
condition and return to the initial state, or die.
Changes of the Damaged Cell
Cytoplasm
The cytoplasm of a
damaged cell loses the ability to form granules. Particles of the dye, entering
the cytoplasm of the normal cell, are surrounded by the membrane to mark
granules. If the cell losses the ability to make granules, its cytoplasm
becomes stained diffusely.
Nucleus Changes
The nucleolemmal cistern
of the nuclear membrane shows the edema and dilation. Chromatin is coagulated
and condensed to form clods. It is called pyknotic nucleus. The regulation of
the protein synthesis is disturbed. The nucleus breaks to form fragments
(karyorhexis). At last it is dissolved (karhyolysis).
Mitochondria Changes
At first mitochondria
shrink, then swell and round, their cristae reduce; synthesis of ATP in them is
low. Finally the membranes of mitochondria breaks, then matrix mixes with
hyaloplasm.
Changes in the Golgi complex
The cisterns of the Golgi complex
divide to form fragments.
Changes in the Lysosomes
The quantity of
lysosomes increases. Membranes of the primary lysosomes are ruptured. The
enzymes of the lysosomes destroy the cell.
The breach of the
permeability of the cellular membrane brings the disturbance of the structure
and function of the organelles and of cell metabolism. Owing to lipids,
proteins and glycogen are stored in the cell cytoplasm. This causes cell
dystrophy developing.
If the intensity and
exposure to harmful agents were short and weak, the cell changes may be
restored. Sometimes the cell structure and function are restored fully. This
cell goes on performing its functions. In other cells structures are restored
incompletely, cells die after a short life span.
Malignant Cells
The genes of DNA
regulate cell division. The failure of regulation leads to uncontrolled
multiplication of cells to form neoplasm. The neoplasm cells receive relative
autonomy and ability to unrest multiplication and metastasis. The daughter
malignant cells receive properties of their precursors resulting in the rapid
growth of the tumor.
Cell Necrosis and Apoptosis
Cell necrosis occurs
after its damage that leads to disturbance of the permeability of the cell
membranes, dilatation of the cell compartments, damage of the structure and
function of ER, mitochondria, and the quantity of the autophagosomes are
increased, finally the cell is destroyed.
The Apoptosis
What is apoptosis? It is
programmed death of cells. DNA of the cell nucleus contains the death gene. The
latter may be stimulated by two causes: 1) if the cell receives special
proteins or hormones, 2) if it doesnt receive
any regulatory signals.
If special hormones
affect the cell, signal molecule is synthesized (cyclic adenosine
monophosphate) then death program starts. For example: when the number of
glucocorticoids in blood is high then receptors of lymphocytes take this
hormone and the program of cell destruction starts. If the regulatory signals
are absent, the death gene of the cell is activated. For example: when
secretion of the luteinizing hormone stops from the hypophysis cerebry, the
cells of the corpus luteum undergo destruction.
The Mechanism of Apoptotic Cell
Death
DNA of chromosomes is
divided to form fragments after activation of the cell death gene. Chromatin of
the nucleus is condensed to form rough particles of the chromatin applying to
the nuclear membrane. The nucleus is rapture to form fragments (microscopic
nuclei). Every micronucleus is surrounded by the nuclear membrane. At the same
time the cell cytoplasm is divided to form the microscopic cells (apoptotic
corpuscles). Then macrophages phagocytize apoptotic corpuscles.
CAPTER 3
GENERAL HISTOLOGY & EPITHELIAL TISSUES
What is tissue? It is a
private system formed during the phylogenesis consisting of one or several cell
types, derivatives of cells, post-cellular structures, and performing special
functions. What is cell type? It is a series of cells, which are at different
stages of differentiation and origin from one initial (stem) cell. For example,
the epidermis cell type includes a series consisting of 5 cells: 1) basal
keratincyte cells; 2) stratum spinosum of kerainocyte cells; 3) stratum
granular of keratinocyte cells; 4) lucid layer of ceratinocyte cells; 5)
cornified layer scales (post-cellular structures). What are derivatives of
cells? It is striated muscle cells and post-cellular structures. Striated
muscle cells (fibres) are formed from cells called myoblasts. The latter are
fused with each other to form striated muscle cell. Erythrocytes losing nucleus
and organelles are called post-cellular structures.
Classification of tissues
Tissues are classified
into 4 types: 1) epithelia (superficial and glandular, the former is classified
into covering and lining epithelium); 2) tissues of internal medium (connective
tissue, blood, lymph, cartilage, and bone); 3) muscles (smooth and striated
muscles, the latter is divided into skeletal and cardiac muscles), 4) nervous
tissue.
A student, speaking on
the structure of tissues should touch upon 4 aspects: 1) origin of the tissue,
2) its location, 3) the tissue structure,
and 4) the tissue function.
Differentiation of Tissue Cells
During the process of
development tissue cells undergo differentiation. What is differentiation? It
is structural and functional alteration of the tissue cells.
Differentiation is
regulated by determination. What is determination? It is a program of the cell
development coded in genes of DNA of chromosomes. After differentiation the
cell becomes active. There are some types of differentiation.
Temporal Differentiation
It is subsequent (one by
one) alteration of cells within the tissue.
Space Differentiation
This is differentiation
when various types of specializing cells are formed within a tissue.
Biochemical Differentiation
It is differentiation
during which new cells secrete special protein types within the tissue.
Differentiation and Stem Cells
At first stem cells
begin dividing. These are initial cells giving origin to the type cells.
Features of stem cells: 1) they have their constant number
2) they can divide and differentiate. The process of cell differentiation is
regulated by the nervous system, endocrine system, immune system and intra
tissue regulation. Intra tissue regulation is provided by chalons. Mature
tissue cells release chalons; which inhibit young cell development. The cell
development pathway is limited by its (the cell) differentiation. For example,
the first blastomeres may be developed to form the mature individual. It is
called totipotent ability. By further division these cells lose this feature.
Such blastomeres are called losing totipotent ability blasomeres.
Regeneration of Tissues
The most of tissue cells
can generate, i.e. they can restore after the cell death or their damage. There
are various processes of regeneration in different tissues. Therefore
regeneration is divided into some types.
Intracellular Regeneration
It is restoration within
the cell. Cells of the nervous tissue, cardiac muscle cells, salivary gland
cells, liver cells are restored (regenerated) by intracellular regeneration because
they do not contain stem cells.
Cellular Regeneration
This is regeneration
when tissue cells can divide. Such regeneration may occur in tissues, which
contain stem cells (epithelial tissue, blood, skeletal muscle).
Histological Regeneration Type
When destroyed
parenchymal cells are substituted by connective tissue it is called typical
histological regeneration. What is a parenchymal cell? Parenchymal cells are
only present the organs. For example, hepatocytes are contained in the liver
only. Apart from parenchymal cells every organ contains stroma cells (usually
connective tissue cells).
Organ Regeneration Type
If parenchymal cells are
formed instead of the destroyed ones, it is called organ regeneration type.
Physiological Regeneration Type
If natural
(physiological) death of the cell, which is substituted by a new parenchymal
cell, it is called physiological regeneration type.
Reparative Regeneration type
It may occur only after
damage to the cell.
Regeneration and Stem Cells
Stem cells are located
densely in some tissues (intestinal crypts) while in other tissues they are
situated diffusely (epidermis of the skin). The tissues regenerate unequally.
It depends on stem cells presenting. If the tissue contains mature cells only,
then the reparative typical organ regeneration cannot occur in this tissue
(nerve tissue, cardiac muscle, sustaining epithelial cells of convoluted
seminiferous tubule). In this tissue cells only intracellular regeneration may
occur. It is necessary to provide
normal structure of the cells. It is
very important because tissue vitality depend on the structure of tissue cells.
EPITHELIAL TISSUES
Epithelial tissues are
classified into glandular and superficial ones. The latter include covering and
lining epithelia. Covering epithelium covers the body (epidermis); lining
epithelium covers lumen surfaces of organs (stomach, urinary bladder etc.).
Glandular epithelium constitutes the glands, which discharge secretions,
hormones and enzymes.
Superficial Epithelium
It is found on the
boundary between the outer and inner environment of the body. It performs
defense, barrier, receptor and metabolism functions. For example, intestinal
epithelium absorbs food; waste substances are discharged through renal
epithelium.
There are 6 features of
the superficial epithelium. 1. The epithelium forms layers. 2. It lies on the
basal membrane, consisting of amorphous substance including proteins, lipids,
carbohydrates, fibronectins, laminins, and fibrils containing collagen of IV
type. Basal membrane consists of light and dark layers, performing barrier,
nourish, metabolic, morphogenesis functions. There is a connective tissue under
the basal membrane. 3. Intercellular substance is absent between epithelial
cells therefore they lie close to one other and are connected by intercellular
junctions (tight junction, denticulate junction, desmosomes etc.). 4. Blood
vessels are absent between epithelial cells, because foods pass through the
basal membrane from connective tissue to the epithelium. 5. Epithelial cells
have the cellular base and the cellular apex, the former is directed towards
the basal membrane, the latter is directed upwards. The basal part of the cell
membrane may form folds, the lateral
part of this membrane has inter-cellular junctions, the apex pole may bear
microvilli, the latter can sometimes form striated border. 6. The epithelium
possesses a high ability to regenerate.
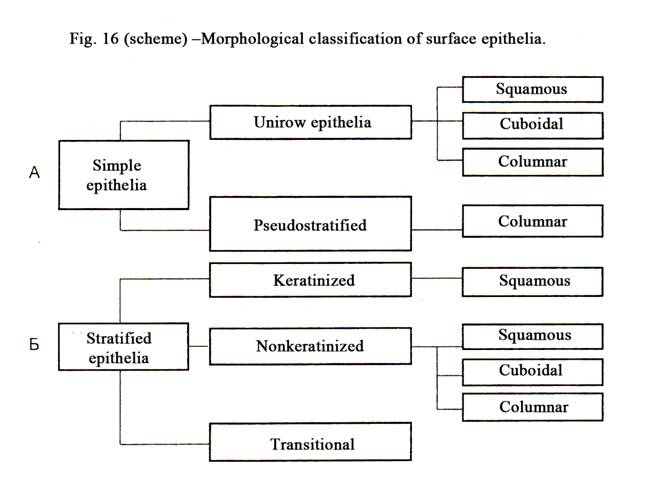
Classification of Superficial
Epithelia
They are classified into
2 singles 1. The division of epithelial tissues depend on them structure and
localization in relation to the basement membrane. It is called morphologic
classification. 2. The division of epithelia depends on their origin. It is
called phylogenic classification.
Morphologic Classification
Superficial epithelium
is divided into simple epithelium and stratified epithelium (Fig. 16).
Simple epithelium
This epithelium is also
divided into unilayered and pseudostratified epithelium.
Unilayered epithelium
is divided into
simple squamous, simple cuboidal and simple columnar epithelium.
Pseudostratified epithelium is always columnar (Fig.17).
Stratified epithelium
It is divided into
stratified squamous keratinized epithelium, stratified squamous nonkeratinized,
stratified cuboidal and columnar epithelium and finally transitional epithelium
(Fig. 17). Calling of the stratified epithelium depends on the shape of the
superficial cells. If they are flat, this epithelium is called squamous. If the
superficial cells are cuboidal or columnar, the epithelia are called cuboidal
or columnar respectively.
The simple epithelium
differs from the stratified epithelium in that all cells of the former lie on
the basal membrane but as for the cells of the latter only one layer of them
lies on the basal membrane, but other layers are stacked over each other.
Phylogenic Classification of
Epithelia
There are 5 types of epithelia. 1.
Epidermal epithelium is derived from ectoderm (epidermis of skin). 2. Endoderm epithelium is developed from
endoderm (the epithelium lining stomach, intestine). 3. Mesoderm epithelium
lining the pleura, renal tubules etc. 4. Ependimal epithelium is derived from
nerve tube (the epithelium lining the central canal of the spinal cord and
ventricles of the brain). 5. Mesenchymal epithelium lining blood and lymphatic
vessels, and heart chambers.
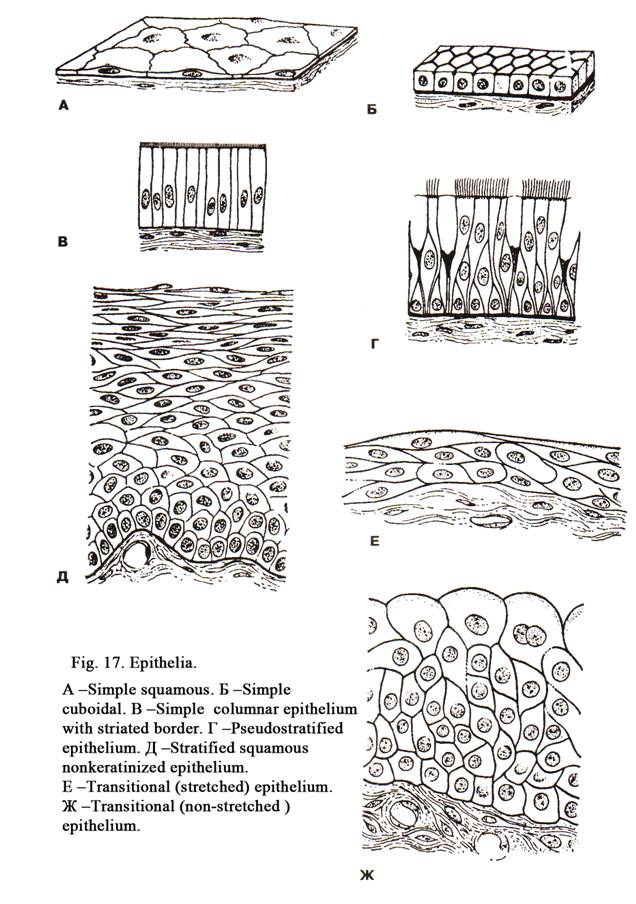
Simple squamous epithelium (Fig.
17A)
It is classified into
endothelium and mesothelium. Endothelium is derived from mesenchyme. It
lines the lumen of the heart chambers, blood vessels and lymphatic vessels.
Endothelial cells have an irregular flat shape; margins of the cells are
indented. The cell contains one or more flattened nuclei, it contains a few
organelles and many vesicles. The surface of endothelium bears short
microvilli. The endothelium is concerned with the exchange of various
substances between blood vessels and surrounding tissues. If the endothelium is
damaged, the thrombus is formed.
The mesothelium is derived from mesoderm, it liens
the pleura, pericardium and peritoneum. The mesothelial cells have flat shape
with indented margins. The cells contain one or two nuclei. The cell cytoplasm
contains a small number of organelles, while it contains many pinocytotic
vesicles. It means that cells take part in the exchange between the serous lumen
and surrounding tissues. The luminal surface of the mesothelial cells bears
many microvilli.
Mesothelium provides
smooth surface of the organs. If mesothelium is damaged, the commissure is
formed and the movement of organs is distorted.
Simple Cuboidal Epithelium (Fig. 17Į)
It lines renal tubules,
excretory ducts of a liver etc. The cells have the cuboidal shape, spherical
nuclei, mitochondria, ER, the Golgi complex and lysosomes. Free surface of
renal epithelial cells bear microvilli to form striated border including
alkaline phosphatase. The basal part of the cell membrane of renal epithelial
cells has basal folds, between the latter mitochondria are located. Striated
border provides absorptive function while basal folds absorb the water from
renal tubules. Renal epithelium is derived from the mesoderm.
Simple Columnar Epithelium (Fig.
17B)
It is present in the
small and large intestine and the stomach. The columnar epithelium of the
stomach lining is derived from endoderm. The cells have the columnar shape,
ovoid nucleus, light cytoplasm, prominent smooth ER, the Golgi complex and
mitochondria. The apical part of cells includes granules containing a mucous
secret. Thus, superficial epithelium of the stomach mucous membrane is
glandular epithelium. This provides 3 functions: 1) synthesis of mucous
secretion covering the mucous membrane of the stomach, 2) the mucous secretion
protects the mucous membrane from chemical and physical affect, 3) the
superficial epithelium of the stomach absorbs water, glucose, alcohol etc.
Simple Columnar Epithelium with
Microvilli
This lining mucous
membrane of the small and large intestines is derived from the endoderm. The
cells are columnar in shape. The cells are joined with one other by tight
junction or zonula occludens. The cells contain prominent organelles and
microfilaments, forming cytoskeleton, desmosomes, and denticulate intercellular
junctions. Superficial surface of the cells bears microvilli. These are 1 mm in length and 0.1mm in diameter. The distance between
the microvilli is about 0.01mm. The
microvilli constitute a striated border. The latter performs 2 functions: 1)
digestion, 2) absorption of digestive substances. Thus, if simple epithelium
has striated border, it means, that it provides the absorptive function. Among
epithelial cells, lining mucous membrane of the small and large intestines,
there are goblet cells, discharging mucous secretion, endocrine cells,
releasing hormones, undifferentiated (stem) cells, providing substitution of
all epithelial cells of the intestine (in 5-6 days). Acidofil granular exocrine
cells are also present. Stem cells of the intestine are localized densely.
Pseudostratified epithelium (Fig. 17Ć)
It is simple epithelium
because its cells lie on the basal membrane, but the cells are various size and
shape therefore the nuclei of the cells lie in different distance from the
basement membrane to form some rows of the nuclei. The nuclei of the smallest
cells lie nearest to the basal membrane, while the nuclei of the largest cells
(ciliated cells) lie furthest of it. The
pseudostratified epithelium is located in the trachea, bronchi, nose cavity,
male deferent duct.
The pseudostratified
epithelium of the respiratory ways includes 4 types of cells: 1) ciliated cells,
2) basal (undifferentiated) cells, 3) goblet cells, and 4) endocrine cells.
Ciliated epithelial
cells (the tallest
cells) of the mucous membrane of the respiratory ways contain nuclei of the
elongated shape and main organelles. The basal narrow end of these cells is
connected to the basal membrane, while the wide cellular apex bears cilia 5-10mm in length. Every cilium contains 9
pairs of peripheral tubules and 1 pair of central tubules (axoneme). The
axoneme is connected to the basal body (changed centriole). The cilia lining
the epithelial surface move like a wave, resulting in fluid, mucous with
trapped dust movement from the trachea and bronchi towards the pharynx.
Uterine tubes are lined
by ciliated epithelium, but it is not pseudostratified epithelium.
Basal epithelial cells
of the respiratory ways are short, their basal part lies on the basal membrane.
These cells can multiple and substitute dead epithelial cells. The basal (stem)
cells lie sparsely in the trachea, bronchi, nose cavity, and epidermis of the
skin. Goblet cells are unicellular glands. They are columnar shape. Their
nucleus is flattened they contain a smooth ER, the Golgi complex and
mitochondria. When secretory granules are stored within the apical part of the
cells, they assume a particular (goblet) shape. Goblet cells provide an
important function. They discharge mucous secretion, which protects mucous
membranes of the trachea and bronchi from physical and chemical exposures.
The endocrine cells discharge noradrenalin and serotonin.
They regulate function of the muscle tissue of the trachea and bronchi.
Stratified Squamous Non-keratinized
Epithelium (Fig.17Ä)
This epithelium lines the mucous membranes of
the oral cavity, esophagus, and cornea of the eye. The epithelium of these
organs is derived from ectoderm. The epithelium is made up of three layers: 1)
basal layer, 2) intermediate layer, and 3) superficial layer.
The basal layer rests on the basement membrane. The
cells are columnar in shape. They are connected with one another by desmosomes
being joined with the basement membrane by hemidesmosomes. Amongst the basal
cells there are stem cells, which are divided to form daughter cells. Part of
the daughter cells rises at the intermediate layer.
Cells of the
intermediate (spinous) layer form some rows. They are irregular in shape. Their cell bodies and
nuclei moving upwards become more and more flattened. The spinous processes
arise from these cells. These processes are joined with those from other cells.
There are desmosomes between spinous processes. The cells become mature and
pass towards the surface of the epithelium to form the superficial layer. Cells
of this layer are flat. The cells lose desmosomes and are removed from the
epithelial surface. The stratified squamous
non-keratinized epithelium
protects deep tissues from
physical and chemical exposure and in addition to absorb some substances and
drugs.
Stratified Squamous Keratinized
Epithelium
This is derived from
ectoderm and covers the surface of the body. It is called epidermis. There are
two types of epidermis: thick (on the palms and soles) and thin (the rest of
epidermis). Thick epidermis contains 5 layers: 1) basal layer, 2) spinous
layer, 3) granular layer, 4) lucid layer, and 5) cornified layer (Fig. 18).
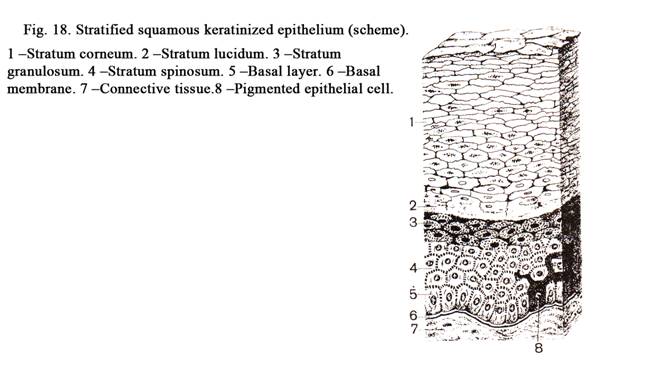
The basal layer (Fig.
18. 1) consists of 4 cell lines: 1) keratinocytes (85%), 2) pigment cells
(10%), 3) Merkels cells, and 4) antigen presenting cells.
Keratinocytes are columnar in shape. They include
prominent organelles, elongated nuclei, and basophilic cytoplasm containing
much RNA. Their cell cytoplasm contains many microfilaments, which undergo
keratosis. The cells are joined with one another by desmosomes and with the
basal membrane by hemidesmosomes. The basal epithelium layer contains stem cells
that undergo mitosis to give off keratinocytes. Some of these cells rise
towards the spinous layer. They can divide. Therefore both the basal layer and
the spinous layer together are called the germinal layer (germinal stratum).
Pigment cells form the second line of
cells they are derived from the neural crest. The cells are star shaped with
long branching processes. They contain a few cell organelles and light
cytoplasm. The pigment cells have not desmosomes (lie loose). Within the cell
there are 2 enzymes: 1) DOP-oxidize and thyrosinaze. These take part
in the process of melanin synthesis
from amino acid tyrosine. Melanin granules are discharged by pigment cells and
ingested by epithelial cells.
Merkels cells are of the neural crest origin
their size is larger then that of epithelial cells. The cytoplasm of the cells
is light. These cells provide the sensitive function.
Dendritic cells of
Langherhans (antigen presenting cells) are derived from blood monocytes. They are
star shaped. The cells contain all organelles but the most prominent organelles
are lysosomes. The dendritic cells ingest waste 4) (antigens, bacteria). These
cells along with lymphocytes constitute the immune system of the dermis.
Stratum Spinosum (Fig. 18. 4)
It consists of some rows
of irregular shape cells. The cells have spines, which are joined with those of
others cells. Between the spines there ate desmosomes. The spines contain a
number of fibrils (made up of keratin filaments) consisting of fibrillar
proteins. Close to the granular layer the spinous cells assume a flattened
shape. Their cytoplasm contains keratosomes, which include lipids. Processes of
Langherhans cells and pigment cells are present in the spinous layer.
Stratum Granulosum (Fig. 18. 3)
The granular layer
consists of 3-4 rows of irregular-shaped cells containing deep stained nuclei.
Organelles of the cells are not prominent. In these cells fillagrin and
keratolaminin are synthesized. Nuclei and organelles of the cells begin
destroying. The cells contain keratohyalin granules. The granules include
keratin, filagrin, and splitting products of the nuclei and organelles. The
keratolamimin attaches to the cell membrane, which becomes thick.
Keratosomes contain
lipids and enzymes. The keratosomes enter spaces between the cells to form a
glue-like substance, which holds the cells of the granular,
lucid, and cornified layers
together. The outermost cells of the granular layer enter the next layer (lucid
layer).
Stratum Lucidum (Fig. 18. 2)
The lucid layer cell nuclei
are destroyed, they may be ruptured or undergo lysis (karhyolysis). The
keratohyalin granules fuse with one another to form a homogenous mass including
microfilament fascicles, which are held together by the filagrin. The formation
of the lucid layer is the next phase of epithelium keratosis. Dies do not stain
the lucid layer while at the same time it refracts rays actively therefore it
is called the lucid layer. When lucid layer cells undergo further
differentiation they enter the next (cornified) layer.
Stratun Corneum (Fig. 18. 1) Layer
The cornified layer is
made up of flattened scale-like elements containing filaments embedded in
protein. The cell nuclei are absent instead of them there are vesicles of air. The
scales are filled with keratin. The scale cell membrane becomes thick because
keratolaminin is fused into it. The scales are held together by glue like
material containing lipids. As the glue like material is destroyed the
superficial scales shed off. The superficial epidermis layers are, constantly
removed and are replaced by proliferation of the basal layer cells. This
process lasts 10-30 dyes. The stratified epithelium provides the barrier,
protective and exchange functions.
Transitional Epithelium (Fig. 17Ę)
It covers the urinary
organs, and is derived from mesoderm. The epithelium consists of 3 layers:
basal, intermediate, and superficial. Basal layer cells are small and dark, the
cells of the intermediate layer are light pear shaped; superficial layer cells
are the largest and umbrella shaped. The latter are joined with one another by
tight junctions and zonula adherens. This type of epithelium is called
transitional epithelium (being
transitional between simple
epithelia and stratified
squamous epithelium). In the urinary bladder the epithelium can be
stretched considerably, when it is filled with urine. At this time the
epithelium become thin and superficial cells become flattened. When the urinary
bladder is empty the epithelium become thick and superficial cells become
round. The epithelium provides the barrier function (prevent passing the urine
through the wall of the urinary bladder).
THE GLANDS (GLANDULAR EPITHELIUM)
Some epithelial cells
may be specialized to perform a secretory function. Aggregations of the cells
form glandules. There are exocrine and endocrine glandules. The former pour
their secretions on the epithelial surface or into the cavity of the body. The
endocrine glands discharge its secretions into blood or lymph. The glands may
be small and included within others organs (the stomach, esophagus, trachea,
bronchi), while they may be very large (the liver).
Usually the process of
secretion of the glandular cells is divided into 4 phases: 1) entering initial
products for secretion, 2) synthesis and storage of the secretion, 3)
elimination of the secretion, and 4) restoration of the cells after the
secretion elimination (Fig. 19).
During the first
phase the initial products (water, amino acids, proteins, carbohydrates,
salts) pass from the capillaries through their basal membrane to the gland
cells.
During the second
phase the entering substances are affected by endoplasmic reticulum to form
new products. Then the products travel to the Golgi complex and undergo
processing within its cisterns to form vesicles. After it the vesicles separate
from the cisterns and are stored in the apical part of the glandular cells.
During the third
phase the secretory granules are discharged from glandular cells. There are
3 types of secretion: a) merocrine, b) apocrine, and c) holocrine (Fig. 19). In
the merocrine type secretory granules are discharged by exocytosis (the cell
remaining intact). During the apocrine secretion the apical part or cell
microvilli are shed off to discharge the secretion. By the holocrine type of
the secretion the entire cell disintegrates while discharging its secretion. An
example of the merocrine secretion is seen in salivary glands, the apocrine
secretion - in atypical sweat glands and mammary glands, and the holocrine
secretion - in sebaceous glands of the skin.
During the fourth
phase the destroyed parts of the cells are restored. After the merocrine
secretion the restoration is not needed, after the apocrine secretion the
apical part of the cell and microvilli are restored, after the holocrine
secretion all destroyed cells are replaced by multiplication of
non-differentiation cells lining the basal membrane.
There are glands, which
cells discharge their secretion diffusely (the adrenal cortex).
Endocrine Glands
The secretion of the
endocrine glands is called the hormone. It is discharged in blood or lymph.
Therefore the excretory duct is absent in them while they are supplied by blood
and lymph vessels better than the exocrine glands. Endocrine
glands are thyroid, parathyroid, and
suprarenal glands, hypophysis and epiphysis cerebry.
Exocrine Glands
Every exocrine gland
consists of the secretory element or terminal region and excretory duct. These
glands discharge their secretion on the epithelial surface or into organ
cavities (the stomach). Exocrine glands are large salivary glands (parotid,
submandibular, and sublingual glands), small salivary glands (lip, cheek,
lingual, and palatine glands), stomach, esophageal, and intestinal glands.
Exocrine Glands Classification on a
Basis their Structure
The exocrine glands are classified
into simple and compound glands (Fig. 20). When single terminal region of the
gland discharges its secretion into single duct this gland is said to be the
simple gland. There can be a number of terminal regions, each terminal region
discharges secretion into its own duct. These ducts unite to form larger ducts,
which ultimately drain on to the epithelial surface. Such gland is said to be
the compound gland. If one terminal
region discharges its secretion into a single duct it is called a simple
non-branched gland. If some terminal regions discharge their secretion into a
single duct, this is called a simple branched gland. If in every duct of the
compound gland many terminal regions discharge their secretion, it is called a
compound branched gland. If in every duct of the compound gland one terminal
region only discharges its secretion,
it is called a compound non-branched gland. If the
terminal region of a gland is tube shaped, it is called the tubular gland
(simple or compound). If the terminal region of a gland is vesicle shaped, it
is called an alveolar gland (simple or compound). If the compound gland
includes both tubular and alveolar terminal regions, it is called a compound
tubular-alveolar gland.
Classification of Glands according
to the Nature of their Secretions
If the gland releases
mucous secretion, it is called a mucous gland. If the gland discharges serous
secretion, it is called a serous gland. If the gland releases both mucous and
serous secretions, it is called a seromucous gland.
Classification of Glands according
to the Manner in Which Their Secretions is Released out of the Cells
If the secretion of an exocrine
gland is released out of the cell by exocytosis, the gland is called a
merocrine gland. If the secretion of an exocrine gland is discharged out from
its cells in apocrine manner, it is called an apocrine gland. If the secretion
of the gland sells is released in holocrine manner, this gland is called a
holocrine gland.
Thus exocrine glands are
divided into merocrine, apocrine, and holocrine.
If glands are derived
from skin (salivary, sweat, sebaceous, mammary glands), their excretory ducts
are lined with stratified squamous epithelium. These glands contain
myoepithelial cells. They occupy the space between secretory cells and the
basal membrane. When myoepithelial cells contract, a secretion is discharged
from secretory cells.
CHAPTER 4
BLOOD AND LYMPH
Blood is the part of
blood system. The system includes 1) blood, 2) blood formation organs, and 3)
lymph. All components of the blood system are derived from mesenchyme. The
blood is within blood vessels and the heart; the lymph is in lymphatic vessels.
Blood formation organs are bone marrow, thymus, lymphatic nodes, the spleen,
and lymph nodes of mucous layers of the gut, respiratory pathways and other
organs. All components of the blood system are associated with one another by
origin and functionally. The genetic association is in that all of them are
derived from mesenchyme. The functional associating is more complete. Every day
some millions of blood cells are destroyed. At the same time the same quantity
of blood cells is created.
A constant level of blood cells is regulated
by the nervous and endocrine systems, surrounding cells and self-regulation.
What are surrounding cells? They are stroma cells and macrophages surrounding
maturating blood cells. The surrounding cells release hemopoietins, which
stimulate blood formation. What is blood self-regulation? The mature blood
cells can discharge inhibitory factors (chalons), which inhibit the growth of
their precursors.
There is a close contact
between blood and lymph. For example, a connective tissue contains fluid
component (ground substance). The latter is filtered from the walls of blood
vessels water, proteins and other organic and non-organic substances. Near
blood capillaries there are lymphatic capillaries. From the walls of the latter
components of ground substance pass into the lymphatic capillary lumen to form
the lymph.
Blood cells can leave
blood capillaries and enter lymphatic capillaries then return from the latter
to the former.
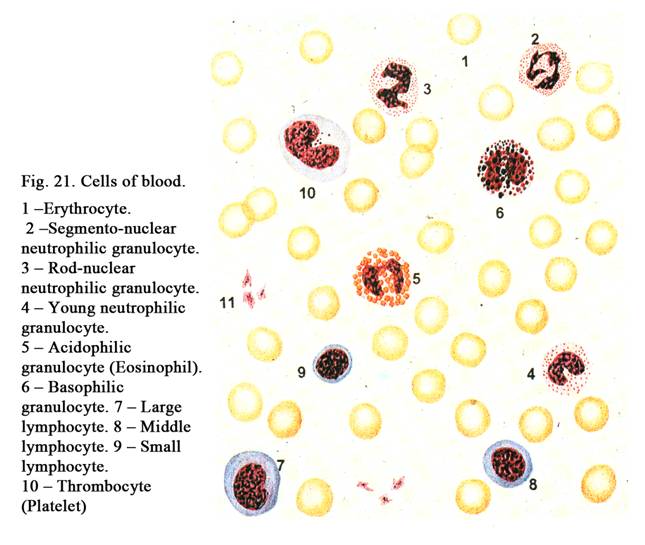
There is a close contact
between the lymph and blood formation organs (lymph nodes). The lymph is
transported from lymphatic vessels to lymphatic nodes.
Bacteria and other
harmful substances are removed from the lymph by macrophages of lymphatic
nodes. Moreover lymphocytes enter the lymph from lymphatic nodes. Then purified
and abundant by lymphocytes the lymph leaves lymphatic nodes and enters the
jugular veins.
Thus, there is a close
connection between blood, connective tissue, and lymph because the exchange
between blood and lymph may only occur through connective tissue.
The Structure of Blood
The blood is regarded as
modified connective tissue because cellular elements in it are separated by an
intercellular substance called the blood plasma: and because some blood cells
have close affinities to cells in general connective tissue. The blood cells
are erythrocytes, platelets, and leucocytes (Fig. 21). Blood is stained with
asur and eosin. In other tissues the internal medium (connective tissue) is of
semi-fluid consistence or dense (cartilage, bone) consistence.
Depending on
intercellular substance these tissues provide various functions. Blood
provides nutrition and protective functions (phagocytosis), cartilages and
bones provide mechanical support of the body and mechanical protection for some
organs (the brain).
The quantity of blood
cells is 40-50%; the rest is plasma. The body blood volume is 5-9%.
Basic functions of blood
are 1) transport, 2) respiration, 3) nutrition, 4) protection, and 5) the
homeostatic function.
Blood plasma contains
90-93% of water, 6-7.5% of proteins (albumin, globulin, fibrinogen); other
substances (organic and non-organic substances) constitute 2.5-4%. Blood plasma
without fibrinogen is called serum. Normal pH of plasma is 7.36.
Erythrocytes
The quantity of
erythrocytes per liter of the male blood is 4.5-5.5 x 1012 the
female blood contains 3.7-5 x1012 erythrocytes per liter. Elevation
of the number of erythrocytes in blood
is called erythrocytosis. Erythrocyte content decrease is called
erythrocytopenia.
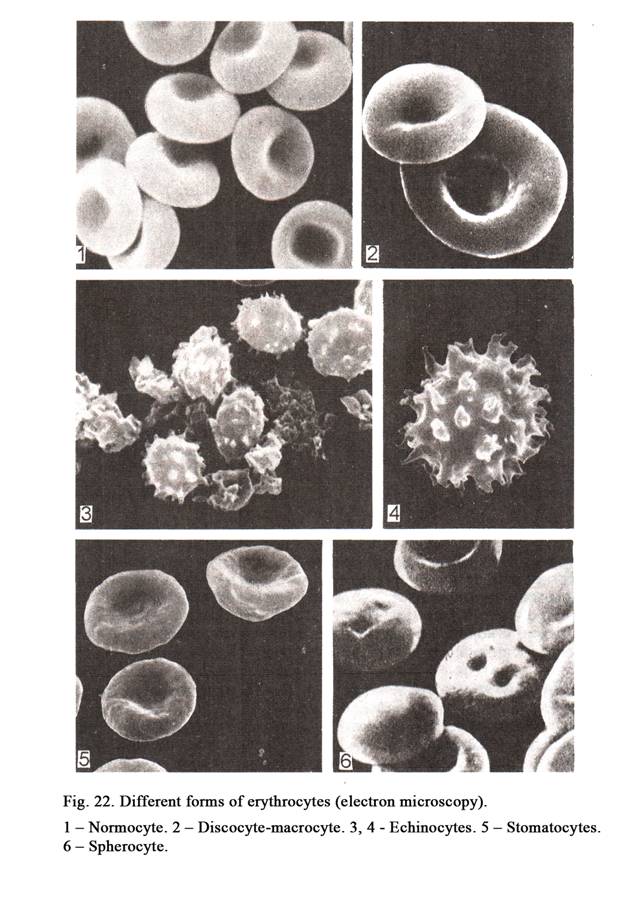
The Shape of Erythrocytes
The erythrocyte is a
biconcave disc (disc cell). The quantity of disc cells is 80%. The thickness of
the disc cell center is 1 mm the
peripheral part thickness is 2-2.5 mm. Erythrocytes may be variable in shape. There
are sphere-shaped erythrocytes, echinocytes etc (Fig. 22.). Sphere-shaped cells
and echinocytes are cells, which life span is ending.
The Size of Erythrocytes
The size of disc cells
is variable in diameter. 75% of disc cells are 7-8 mm in diameter (normal erythrocytes),
12.5% of cells are 4.5-6 mm in
diameter (small erythrocytes), and 12.5% of disc cells are 8-10 mm in diameter (large erythrocytes).
The Structure of Erythrocytes
Erythrocytes are not
cells because the nucleus and organelles are absent in them. The plasma
membrane of erythrocyte is 20 nm. On the surface of the plasma membrane
glycoproteins, amino acids, proteins, enzymes, hormones, drugs, and others
substances are localized. On the lumen surface of the plasma membrane there are
glycolysis enzymes, Na+-ATP, K+-ATP, and hemoglobin.
The plasma membrane
consists of lipids (45-47%), proteins (45-47%), and glycoproteins (5%). Lipids
form 2 layers of lipid molecules. The external lipid layer includes
phosphatidylholine and sphingomyelins; the internal layer consists of
phosphatidilserine and phosfatidyl-ethanolamine.
Proteins include
intramembrane proteins (glycopharin, protein of stripe 3) and sub membrane
proteins (spectrin, protein of stripe 4.1, actin, and ankyrin), inner membrane
protein, and outer membrane protein (Fig. 23).
The internal end of
glycopharin is joined with the «nodal complex», its external end passes through
2 lipid layers of the plasma membrane to the external surface of the latter to
form the receptor of the erythrocyte.
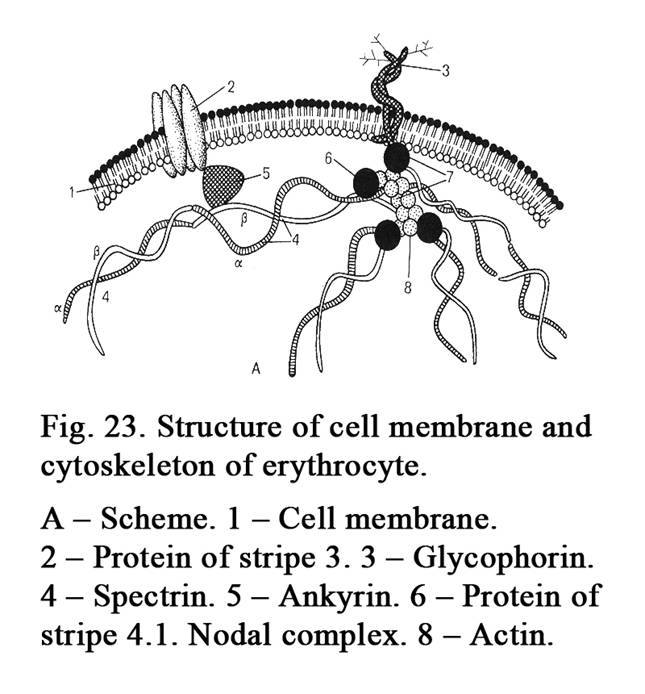
Protein stripe 3 is a
polypeptid chain, which passes repeatedly to and from the plasma membrane
surface to form hydrophilic pores. The latter provide anions HCO3-
and Cl- passageway when O2 is joined with an erythrocyte.
At the same time anion HCO3- is replaced by anion Cl-.
Sub membrane protein
spectrin is a filament (its length is about 100 nm) consisting of 2 polypeptid
chains (a-spectrin and b-spectrin). The proximal end of the
spectrin is connected to actin filaments, present within the «nodal complex»
while its distal end is joined with ankyrin; the latter is connected with
protein stripe 3.
The «nodal complex»
consists of actin, protein stripe 4.1 and ends of spectrin and glycopharin.
Glycolipids and
glycoproteins form glycocalyx. The presence of ABO antigens on the surface of
erythrocytes depends on glycolipids and glycoproteins. On the erythrocyte
surface there are 2 types of antigens (A and B). The blood plasma contains 2
substances (a &b). If the «foreign» antigen «A» meets substance
a or antigen «B» meets substance b erythrocytes are joined
(agglutination).
BLOOD GROUPS
There are 4 blood groups
depending on antigens of erythrocytes and a and b substances of blood plasma.
Group I (0) antigens A
and B are absent, both substance a and substance b are present.
Group II (A) antigen A
and substance b are
present.
Group III (B) antigen B
and substance a are
present.
Group IV (AB) antigens A
and B are present, both substance a and b are absent.
Most people (86%) are
called rhesus-positive. Their erythrocytes bear the Rhesus factor. The rest of
people do not have the Rhesus factor. They are called rhesus-negative. If the
rhesus-positive blood is transfused to a patient with rhesus-negative blood,
the haemolysis occurs because rhesus antibodies are formed. The excess of amino
acids of blood is absorbed by the plasma membrane of erythrocytes therefore the
contents of amino acids is constant in blood.
The erythrocyte contains
about 40% of dry substance; the rest is water. 95% of the dry substance of erythrocytes
is hemoglobin. The latter consists of the protein called globulin and a
pigment. There are 2 types of hemoglobin (hemoglobin A and hemoglobin F).
Hemoglobin A is adult hemoglobin, hemoglobin F is fetal hemoglobin. After
puberty the quantity of hemoglobin A is about 98%, the quantity of hemoglobin F
is only 2%. The quantity of hemoglobin A of a newborn is 20%; the rest is
hemoglobin F.
After the erythrocyte
death it is engulfed by macrophages in the spleen. Within the macrophage
hemoglobin undergoes splitting to form bilirubine and the substance containing
iron. Iron enters the blood and travels in the bone marrow. Here special
macrophages engulf the iron and give it to new forming erythrocytes. Therefore
these macrophages are called food cells.
The energy source of
erythrocyte is glycolysis, providing erythrocytes by ATP and NAD-H2.
The ATP provides transport of various substances across the cell membrane (K+,
Na+, and many others substances). These ions keep the optimum
balance of pressure within erythrocytes and blood plasma. In addition, ATP
maintains the regular shape of erythrocytes. The NAD-H2 prevents the
hemoglobin conversion into met-hemoglobin. The latter is strong synthesis the
hemoglobin with some chemical substances. The met-hemoglobin cannot transport
oxygen or carbon dioxide. If a person is an active smoker his blood contains
10% of the met-hemoglobin, which is useless for him. Hemoglobin combined with
oxygen and hemoglobin, combined with carbon dioxide are not strong
combinations. The quantity of hemoglobin of normal blood is 120-
In blood there are 1-5%
of young erythrocytes (reticulocytes). They contain remnants of ER, ribosomes,
and mitochondria, which are stained by dyes well. The remnants of organelles of
reticulocytes are seen as reticulum. Therefore they are called reticulocytes.
Hemoglobin in reticulocyte is synthesized by remnants of ER. Reticulocytes
mature within sinusoid of the bone marrow or within peripheral vessels.
The life span of
erythrocytes is about 120 days. After it glycolysis within erythrocytes is
disturbed, ATP and NAD-H2 formation is stopped, the shape of
erythrocytes becomes irregular (echinocytes, sphere like erythrocyte), the
permeability of plasma membrane by Na+ and K+ changes,
therefore the osmotic pressure within erythrocytes becomes high. This leads to
water entering the erythrocyte, which becomes swollen, its cell membrane is
ruptured finally hemoglobin leaves the cell (haemolysis). The normal
erythrocyte can undergo haemolysis if water is infused into blood this leads to
the decrease of osmotic pressure of blood plasma. After haemolysis only the
plasma membrane of the erythrocyte can be seen. The latter is called the shadow
of the erythrocyte.
The impairment in NAD-H2
synthesis leads to the formation of met- hemoglobin. The surface of
erythrocytes shed off sialic acid, which has negative charge, therefore
erythrocytes fuse. Skeletal protein spectrin of old erythrocyte is changed so
it is converted into echinocytes. The plasma membrane of old erythrocytes is
able to catch autolytic bodies IgG1 and IgG2 to form
certain complexes. Such erythrocytes are engulfed by macrophages. Death of the
erythrocytes, usually, occurs in the spleen.
General Features of Leucocytes
The quantity of leucocytes is 4-9x109
per liter. Increase of the number of lymphocytes is called leukocytosis, the
decrease is called leucopoenia. Leucocytes are classified into granulocyte
leucocytes and agranular leucocytes. The
cytoplasm of contains granules (Fig. 24), which are absent in agranular
leucocytes. If the granules of leucocytes are stained by acidic dye (eosin),
they are called eosinophil leucocytes. If the granules of granulocytes are
stained by basic dye (azure), they are called basophile leucocytes. If the
granules of leucocytes are stained both by basic and acidic dyes, the
leucocytes are called neutrophils.
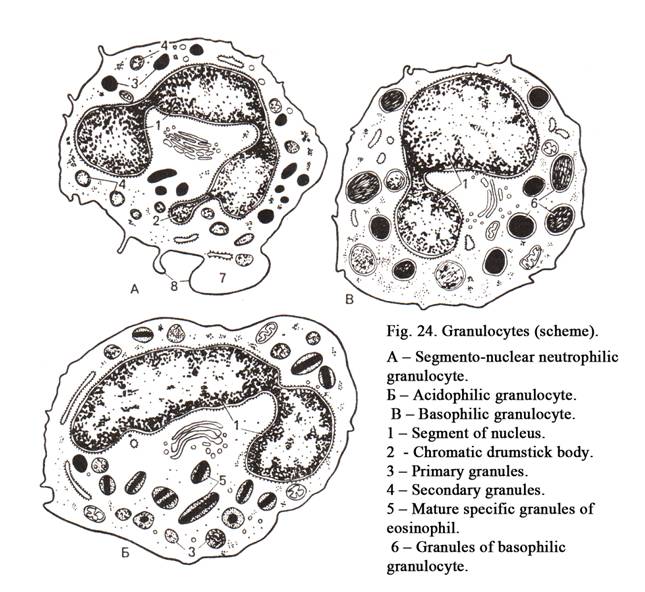
All leucocytes are
spherical, they can move in liquid. Leucocytes can move out of blood to
surrounding tissues where they perform their functions. In fact the blood is
merely a rout by which leucocytes travel from bone marrow to other
destinations. Most leucocytes have a relatively short life span. All leucocytes
perform the protective function.
Neutrophils
When neutrophils are
suspended in blood, they are 7-8 mm in diameter, when they are spread out on
glass slide their diameter is about 12 mm. The cytoplasm of neutrophils contains of 2
types of granules: 1) azurophilic granules or lysosomes, their quantity is
10-20%, and 2) specific granules stained by both basic and acidic dyes.
Azurophilic granules are 0.4-0.8mm in diameter. They contain
proteolytic enzymes having acidic reaction (acid phosphatase, peroxidasa, acid
protease, lysozyme, aryl sulphatase).
The quantity of specific
granules is 80-90%. They contain both acid and alkaline enzymes and
substances (alkaline phosphatase, alkaline proteins, phagocytin, lactoferrin,
lysocyme). Lactoferrin 1) connects molecules Fe with each other and fuses
bacteria, and 2) inhibits the differentiation of young granulocytes. The
peripheral part of neutrophilic cell cytoplasm does not contain granules, here
there are filaments containing contractile proteins. These filaments are
concerned with throwing pseudopodia, which take part in phagocytosis and moving
of cells.
The cytoplasm of neutrophils is stained weakly
acidophilically, lacks organelles. The cytoplasm includes glycogen and lipids.
The neutrophil nuclei have various shapes. According to the nucleus shape there
are 1) young neutrophils (the nucleus is bean-shaped), 2) rod-like neutrophils
(the nucleus is S- or C-shaped), and 3) segmento-nuclear neutrophils (the
nucleus is made up of 2-7 lobes that are joined by delicate strands)
The segmento-nuclear
neutrophilic granulocyte quantity is 47-72%. The cells are called so because they have the
nucleus containing segments (lobes). The nuclei contain heterochromatin, but
nucleoli are absent. A segment of nuclei can throw out sex chromatin, which is
drum stick-shaped. The drumstick is only present in the female blood.
The rod-nuclear
neutrophilic granulocyte contains the nucleus, which is like curved rod (S or C). The quantity
of rod-nuclear neutrophilic granulocytes is 3-5% in peripheral blood.
The number of young
neutrophils is 0-1% in circulation. Their nuclei are bean shaped.
The neutrophils provide
many functions. On the plasma membrane surface they bear Fc and C3 receptors.
They can catch complexes consisting of antigens, antibodies, compliment
proteins and can engulf them. (Complement proteins are a group of proteins,
which are able to destroy antigens). Neutrophils engulfing bacteria discharge
oxidants and lysozyme, which break down the bacteria. After engulfing the
bacteria are digested by enzymes of specific granules then they fuse with
lysosomes, where the bacteria are digested completely. Neutrophils discharge
inhibitors (chalons), which inhibit the proliferation of young granulocytes.
The life span of neutrophils is about 8 days. They are present in blood during
8 hours then they enter connective tissues, where fulfill their functions.
Eosinophil Granulocytes
The quantity of
eosinophil granulocytes is 1-6% in circulation. Suspending in a blood cell is
8-9 mm in diameter on slide glass it is
13-14 mm. Eosinophil granules are only
stained by acid dyes. The form of their granules is oval; their length is 1.5 mm. The granules contain crystalloid.
The outer part of each granule contains the alkaline protein and cationic
protein, and peroxidasa. In these cells there are smaller granules. The latter
contain histamine, aril sulphatase, and factor blocking secretion from
basophilic granulocytes and mast cells. The cytoplasm of eosinophil
granulocytes is weakly stained by basophilic dies it contains not prominent
organelles.
The nuclei of eosinophil
granulocytes may be bean-shaped, rod shaped, and segmented (they contain 2-3
lobes).
Eosinophil granulocytes
inhibit inflammatory reactions; they can engulf foreign substances, release
active oxidants. These cells take part in allergic and anaphylactic reactions
when foreign proteins enter the human body. Eosinophil granulocytes break down
histamine by 4 manners: 1) histamine, released by basophilic granulocytes or
mast cells, is destroyed by histaminase of eosinophil granulocytes, 2) special
factor of the eosinophil granulocytes blocks releasing histamine from
basophilic granulocytes and mast cells, 3) eosinophil granulocytes engulf
histamine, and 4) receptors of these cells catch histamine and hold on own
surface. On the cell membrane of eosinophil granulocytes there are Fc
receptors, which can catch IgE, IgG, and IgM, C3, and C4 receptors.
Active participation of
eosinophil granulocytes in anaphylactic reaction (stroke) occurs when aryl
sulphatase of the cells neutralizes anaphylactic substance released by
basophilic granulocytes.
The life span of
eosinophil granulocytes is some days. In peripheral blood they are present for
about 8 hours.
Increase of these cells
in blood is called eosinophilia; decrease of the cells is called eosinopenia. Eosinophilia
takes place when foreign proteins, hearth of inflammations, and complexes of
antigen-antibodies are present in the organism. A corticotrophin (ACTH),
cortisol, and adrenalin bring about eosinopenia.
Basophilic Granulocytes
The number of basophilic
granulocytes is 0.5-1% in peripheral blood. Suspended in blood the cells are
7-8 mm in diameter, in slide glass they
are 11-12 mm. The cytoplasm contains
metachromatic basophilic granules. The term metachromatic means that the colour
of granules after staining different from the colour of the dye. For example,
azure stains substances in violet colour while those granules are stained in
purple colour. Granules of basophilic granulocytes contain heparin, histamine,
chondroitin sulphate, and hyaluronic acid. In the cytoplasm there are
peroxidasa, acid phosphatase, histidindecarboxylase, and anaphylactic
substances. Histidindecarboxylase is the marker of basophilic granulocytes.
The basophilic
granulocytes nuclei are lobular or ovoid-shaped and their contour is hardly
seen.
If organelles of cells
are weakly developed, the cell cytoplasm is stained slightly. Basophilic
granulocytes act as weak phagocytes. On the cell surface there are E-receptors,
which can hold antibodies. The cell basic function is synthesis of heparin and
histamine found in their granules. These substances regulate local homeostasis.
Histamine increases the permeability of ground substance and capillary wall,
clotting of blood and inflammatory reaction. Heparin provides relaxation of
blood clotting, permeability of capillary wall and inflammatory reaction. When
an antigen influences basophilic granulocyte, the latter discharges the
histamine (degranulation) from its granules. This process increases the
permeability of capillary blood wall and edema of surrounding tissues.
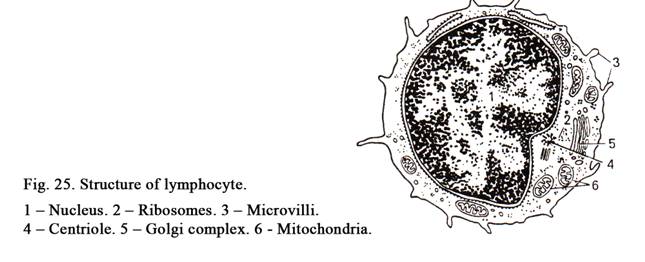
Basophilic granulocytes
play the main role in the development of allergic and anaphylactic reactions.
On the cell surface there are receptors, which can catch IgE.
Agranulocytes
Agranulocytes are divided
into lymphocytes and monocytes.
Lymphocytes
Lymphocytes constitute
19-37%. Deficiency of lymphocytes is called lymphocytopaenia. According to
their size they are divided into small lymphocytes (the diameter is less than 7
mm), middle lymphocytes (they are
8-10 mm in diameter), and large
lymphocytes (their diameter is more than 10 mm). The nuclei (Fig. 25) of lymphocytes are
round-shaped (sometimes may be indented). The cytoplasm is slightly basophilic,
it contains not many organelles; lysosomes are also present.
With the help of
electron microscopy 4 forms of lymphocytes can be seen: 1) small light
lymphocytes constitute 75%, they are 7 mm in diameter, a thin layer of slightly
basophilic cytoplasm surrounds the nucleus, the cytoplasm contains not prominent
organelles (mitochondria, Golgi complex, rough ER, lysosomes), 2) small dark
lymphocytes constitute 12.5%, they are 6-7 mm in diameter, their deep basophilic cytoplasm
layer, surrounding the nucleus, is thinner than that of light lymphocytes,
the former contains many RNA,
ribosomes, and mitochondria (other organelles are absent), 3) middle
lymphocytes constitute 10-12%, they are about 10 mm in diameter, their cytoplasm is slightly
basophilic, it contains ribosomes, ER, and lysosomes, the nuclei of the cells
are round in shape, they ,sometimes, are
indented, include euchromatin and nucleoli, and 4) plasma cells
constitute 2%, they are 7-8 mm in
diameter, their cytoplasm is slightly basophilic, near the nucleus in the
cytoplasm there is a small court, which is less colored and contains the Golgi
complex and the cytocenter, the cell cytoplasm contains prominent rough ER
which surrounds nucleus as a chain. Plasma cells produce antibodies.
Functionally lymphocytes are classified into
B-lymphocytes, T-lymphocytes and 0-lymphocytes (zero-lymphocytes).
B-lymphocytes are formed in bone marrow; they
undergo antigen dependent differentiation in bursa of Fabricius of birds. The
function of B-lymphocytes is production of antibodies (immunoglobulins). The
latter are receptors, which may be concentrated in certain sites of the cell
membrane, may be scattered on the surface, or may by moved along the cell
membrane. Receptors of B-lymphocytes can catch antigens and ram erythrocytes.
T-lymphocytes are classified into T-helpers, T-suppressers, and T-killers. Both T-helpers and T-suppressers regulate the humoral immunity. T-helpers stimulate proliferation and differentiation of B-lymphocytes, and synthesis of antibodies. T-suppressers inhibit proliferation and synthesis of antibodies by B-lymphocytes. T-kil- lers take part in the cellular immunity, i.e. they destroy bacteria, viruses, fungi, and genetic foreign cells. K-cells are also killers, but they kill foreign cells if in the host there are antibodies against them (foreign cells). On the surface of T-lymphocytes there are receptors, which can take and hold mouse erythrocytes.
0-lymphocytes are
undifferentiated cells (they are reserve cells).
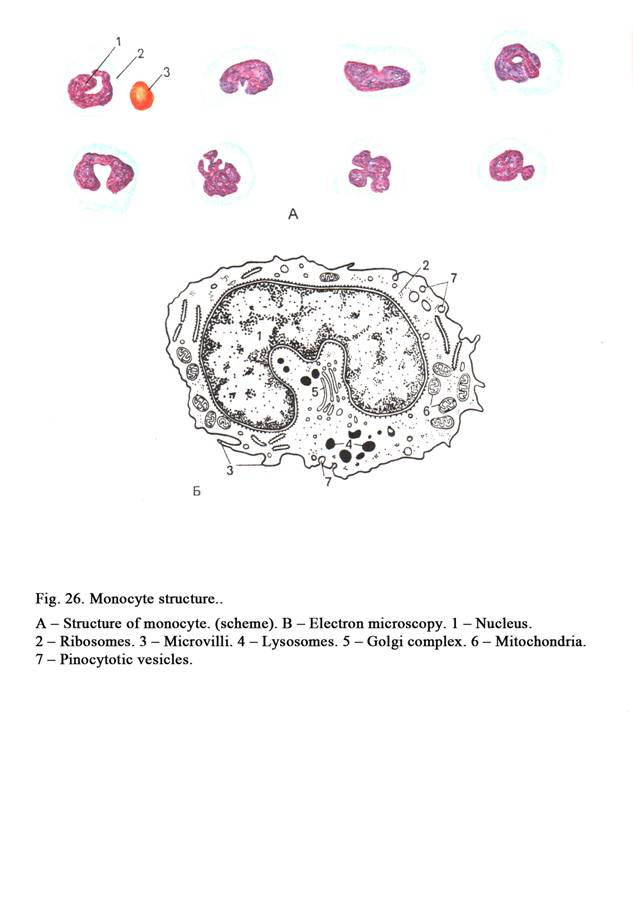
The morphological
structure of B- and T-lymphocytes is similar, but rough ER is better developed
in B-lymphocytes than in T-lymphocytes. B- and T-lymphocytes may be
distinguished by special methods.
The life span of
T-lymphocytes is from some month to some years, B-lymphocytes live some weeks
to some month.
The morphological structure
of stem cells is undistinguished from that of small lymphocytes. If stem cells
enter the connective tissue, they change into mast cells, fibroblasts, etc.
Monocytes
These cells constitute
3-11%, they are 14 mm in
diameter in blood and 18 mm on
slide glass. The cytoplasm of monocytes is slightly basophilic, it contains all
organelles (Fig. 26), but the most prominent organelles are lysosomes. The
nucleus of monocytes is bean-shaped, may be horseshoe shaped, and ovoid. The
function of monocytes is phagocytosis. Monocytes circulate in blood 36-104
hours, and then they enter the surrounding tissues, there undergo
differentiation in macrophages (glial macrophages in nervous tissue, alveolar
macrophages in the lung, star shaped cells in the liver, osteoclasts in bones,
dendrite cells in epidermis of the skin, etc.). At during of phagocytosis
macrophages release oxidants. Macrophages stimulate proliferation and
differentiation of both B- and T-lymphocytes; they also take part in immune
reactions.
Blood Platelets (Thrombocytes)
They constitute 2.5-3.0
x109 per liter. Deficiency of blood platelets is called
thrombocytopaenia. Platelets are particles of the cytoplasm of megakaryocytes.
The particles are 2-3 mm in
diameter. They consist of hyalomere and granulomere (Fig. 27). The former is
the skeleton of the platelet of different substances pass through it. The cell
membrane of platelets bears receptors (glycoproteins). It is PIb receptors;
they catch von Willibrands factors. These are main factors, which provide
blood clotting. Other receptors are PIIb-IIIa; they catch fibrinogen, and also
take part in platelet aggregation.
The cell membrane of platelets is surrounded by thick
(15-20 nm) glycocalyx. The membrane makes invaginations to form the opening
canaliculus system. Platelets of different substances pass through it. The cell
membrane of platelets bears receptors (glycoproteins). It is PIb receptors;
they catch von Willibrands factors. These are main factors, which provide
blood clotting. Other receptors are PIIb-IIIa; they catch fibrinogen, and also
take part in platelet aggregation.
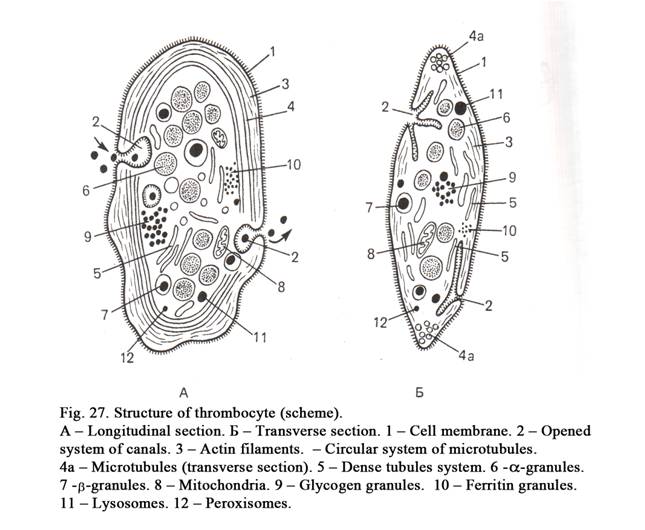
The hyalomere is represented by actin filaments
situated under the cell membrane and bundles of microtubules, which line
circularly near the cell membrane to form the cytoskeleton of the platelet.
Actin microfilaments take part in contraction of the blood clotting volume.
A dense tubular system
of platelet, situated in its center, consists of tubules resembling smooth ER.
On the surface of tubules cytooxygenases and prostaglandin are synthesized, in
their lumen the bivalent cations are joined, and ions of Ca++ are
accumulated. Calcium allows to adhesion and aggregation of platelets.
Arachidonic acid of
dence tubular system is broken up into prostaglandin and thrombaxan A-2, which
stimulates platelet aggregation.
The granulomere includes organelles (ribosomes,
mitochondria, lysosomes, peroxisomes) and components of organelles (ER, Golgi
complex), glycogen, and special granules.
There are 3 types of
special granules.
Type I granules are called a-granules; they are 350-500 nm in
diameter. They contain proteins, glycoproteins, fibrin; platelet derived growth
factor, enzymes.
Type II granules are called b-granules; they are 250-300 nm in diameter.
They are dense corpuscles containing serotonin (5-hydroxytriptamine) coming
from blood plasma, histamine, adrenalin, calcium, ADP, and ATP.
Type III granules are called delta-granules;
they are 200-250 nm in diameter. These are lysosomes, containing lysosomal
enzymes, peroxisomes containing peroxydasa and catalasa.
There are 5 types of
platelets: 1) young, 2) mature, 3) old, 4) degenerative, and 5) giant. The
function of platelets is thrombus formation when blood vessels are damaged.
The process of thrombus
formation: 1) damaged tissues discharge the external factor of blood clotting
and adhesion of platelets, 2) aggregation of platelets and releasing of the
internal factor of blood clotting, and 3) fibrinogen is converted in fibrin to
form blood clotting, vessels are closed and bleeding is stopped.
Aspirin inhibits blood
clotting.
Blood count
It is the number of
formed elements of blood in a certain volume of blood (per liter), the quantity
of hemoglobin per liter, and erythrocyte sedimentation rate (ESR).
Relative
Number of Leucocytes
It is the percentage of
leucocytes in blood. For example, the quantity of segmento-nuclear neutrophilic
granulocytes is 47-72%, the quantity of rod-nuclear neutrophilic granulocytes
is 3-5%, the number of young granulocytes is 0.5%, the quantity of basophilic
granulocytes is 0.5-1%, the number of eosinophil granulocytes is 1-6%, quantity
of monocytes is 3-11%, and the number of lymphocytes is 19-37%. In ill persone
the number of young and rod-nuclear granulocytes is increased. It is called
the blood count has moved left.
Changes in the Number of Blood
Formed Elements in Different Age
One liter of blood of a
newborn contains 6-7x1012 erythrocytes, at the age of 14 days the quantity of the cells is 4-5x1012,
at the age of 6 month the number of erythrocytes becomes minimum (physiological
anemia), in puberty
the quantity of erythrocytes is normal. Age dependent number
of neutrophilic granulocytes and lymphocytes changes significantly. The
quantity of neutrophilic granulocytes and lymphocytes of a newborn is similar
to that of an adult at the age of 4 days the quantity of both neutrophilic
granulocytes and lymphocytes is equal (the first physiological cross). After it
the number of neutrophilic granulocytes decreases, that of lymphocytes increases. At the age of 1-2
years the quantity of lymphocytes is maximum, the number of neutrophilic
granulocytes is minimum. After it the quantity of neutrophilic granulocytes
increases, but the number of lymphocytes decreases. At the age of 4 years the
number of both neutrophilic granulocytes and lymphocytes becomes equal (the
second cross), then the quantity of neutrophilic granulocytes
increases, but the number of
lymphocytes decreases. In puberty the number of neutrophilic granulocytes and
lymphocytes is normal (like in the adult).
The Lymph
The lymph consists of
the lymph plasma (98%) and formed elements of blood. The lymph plasma includes
water, organic substances, and salts. Formed elements of blood are predominantly
lymphocytes (98%); the rest are other cells of blood (2%). Ground substance
undergoes renewing und delivering from bacteria, bacterial toxins and other
harmful substances in lymph nodes. Thus, the lymph contains more lymphocytes,
but fewer proteins than blood.
CHAPTER 5
CONNECTIVE TISSUES
Connective tissues are
internal environment tissues classified into proper connective tissue and
skeletal tissues (cartilage and bone). Proper connective tissue is divided into
1) collagen connective tissue including loose collagen connective tissue and
dense collagen connective tissue, the latter is subdivided into regular type
and irregular type connective tissue, 2) special tissues (adipose, mucoid,
reticular, and pigment).
Loose connective tissue
(Fig. 28) includes cells and intercellular substance, which also contains a lot
of cells and ground substance. Dense connective tissue contains many fibers and
less cells and ground substance.
According to the
quantity of cells and intercellular substance tissues provide different
functions. For example, loose connective tissue is more concerned with trophic
function and less support and mechanical functions while dense connective
tissue is more concerned with support and mechanical functions.
General functions of
connective tissues are 1) trophic, 2) mechanical protective function (skull
bones), 3) supporting and mechanical functions, 4) defence function
(phagocytosis and immunity), 5) formation function (sclera forms the eye
shape), plastic function (adaptation to different condition of the environment,
wound healing), 7) participation in the support of homeostasis.
Loose Collagen Connective Tissue
It includes cells,
ground substance, collagen fibers, elastic fibers, and reticular fibers. Loose
collagen connective tissue lies under the basal membrane, surrounds blood
vessels and lymphatic vessels, it forms organ stroma. It is stained by
toluidine blue.
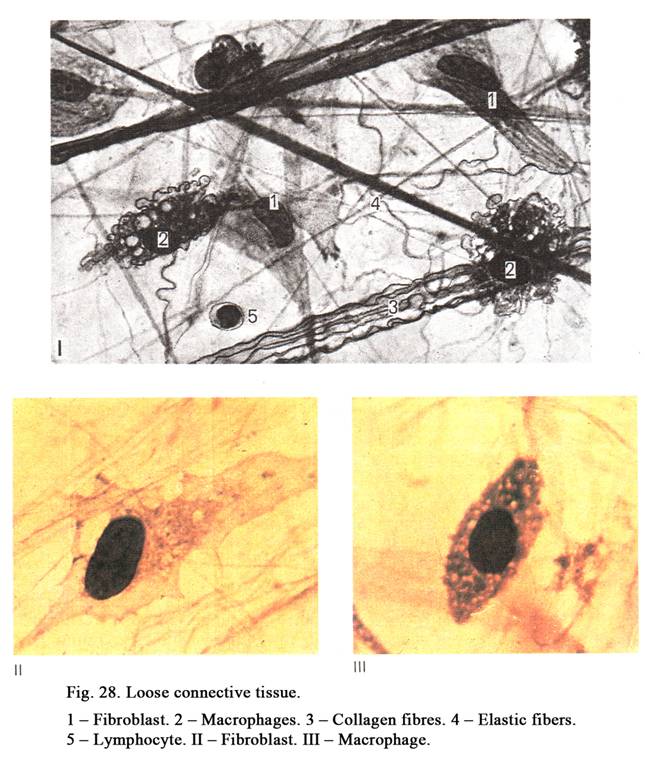
Connective Tissue Cells
The cells of connective
tissue are 1) fibroblasts, 2) macrophages, 3) plasma cells, 4) mast cells, 5)
adipose cells, 6) pigment cells, 7) undifferentiated cells, 8) reticular cells,
and 9) blood leucocytes. Thus, connective tissue consists of some cell lines
(differons). What is a cell line? It is group of cells on different stages of
the development, which are derived from special stem cell.
Line of Fibroblasts (Fig. 28 II)
This line includes stem
cells, precursor cells, undifferentiated and differentiated fibroblasts (Fig.
29), and fibrocytes. The undifferentiated fibroblast is the precursor of
myofibroblast and fibroclast. In embryonic life fibroblasts are derived from
mesenchymal cells, after birth they are derived from stem cells and
undifferentiated cells.
Undifferentiated
fibroblasts are
spindle-shaped cells their length is about 25 mm. The cells have a few outgrowthes their
cytoplasm is basophilic because it contains lot of RNA and ribosomes. The
nucleus of the cells is ovoid; it contains heterochromatin and nucleolus. The
cells are able to multiply by mitotic division and undergo differentiation so
that they are converted into differentiated fibroblasts.
Differentiated
fibroblasts (Fig. 28 & 29) are elongated flattened cells their length is about 50 mm; they have a number of outgrowths.
Their cell cytoplasm is slightly basophilic it contains abundant granular ER
and lysosomes. In the cytoplasm the enzyme, splitting collagen, is reveled. The
nucleus of the cell, staining slightly basophilic, containing euchromatin and
nucleolus, is ovoid. In the peripheral part of the cytoplasm there are
filaments. Owing to the filaments fibroblasts can move in the intercellular
substance.
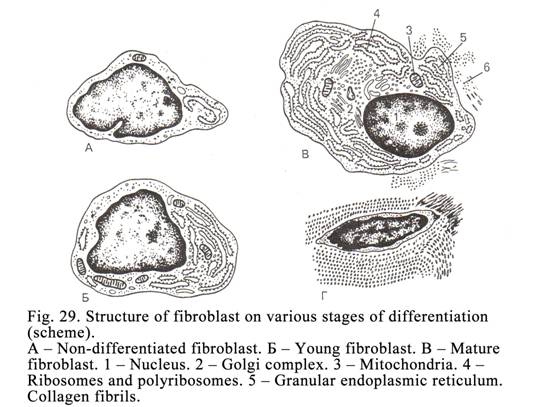
Fibroblast functions: 1) they release molecules of
collagen, elastin, and reticulin (collagen III) from which fibres are formed.
Protein secretion is provided by the entire cell membrane, which takes part in
linking of the fibres, 2) fibroblasts release glycosaminoglycans (keratan
sulphate, heparan sulphate, chondroitin sulphate, dermatan sulphate, and
hyaluronic acid) entering the ground substance, 3) these release glycoproteins
(glue substances), and 4) the cells discharge proteoglycans
(protein-carbohydrate complexes). Fibroblasts can engulf waste substances.
Thus, fibroblasts are cells, which in fact form the connective tissue. If there
are no fibroblasts, there is no connective tissue.
Fibroblasts are active
when vitamin C and compound of Fe, Cu and Cr are present in the organism. If
these substances are absent, fibroblasts are inactive. In this cause collagen
fibres stop renewing; ligaments become weak; glycosaminoglycans of ground
substance are non-formed. It happens in the joining apparatus weakness (for
example dental ligaments) resulting in teeth losing. Because the hyaluronic
acid (main glycosaminoglycans) is absent the permeability of blood vessel walls
is enhanced, and leads to local bleeding. This disease is called scorbutus.
Fibrocytes are formed by further
differentiation of fibroblasts. The nuclei of the cells contain heterochromatin
the nucleoli are absent. The size of fibrocytes is less, than that of
fibroblasts. Organelles of fibrocytes are not prominent their functional
activity is weak.
Myofibroblasts are derived from undifferentiated
fibroblasts. Their cell cytoplasm contains prominent myofilaments, which are
able to contract therefore they provide the contractive function.
Myofibroblasts are present in the uterus wall during pregnancy. This allows the
increase of the uterus myometrium in pregnancy.
Fibroclasts are also derived from undifferentiated
fibroblasts. These cells contain most developed lysosomes, including
proteolytic enzymes, which allow the
break up of the intercellular substance, cells of the uterus wall after
pregnancy, and necrotic material in wounds.
Macrophages
(Fig. 28 III, 30) are derived from blood stem cells and monocytes. They are
present near blood and lymphatic vessels. These cells are ovoid, round or
elongated. They are 20-25 mm in
diameter. There are pseudopodia on the surface of macrophages. The surface of
macrophages is sharply marginated. Their cell membrane bears receptors, which
can catch antigens, immunoglobulins, lymphocytes, and other structures.
The Nuclei of macrophages, containing
heterochromatin, are ovoid, round or elongated. Among macrophages there are
poly nuclear cells (foreign body giant cells). The macrophage cytoplasm,
containing many lysosomes, phagosomes, and vacuoles, is slightly basophilic.
These cells contain moderately developed organelles. The basic fnnction of
macrophages is phagocytosis. Antigens, bacteria, foreign proteins are caught by
macrophage pseudopodia. Lysosomal enzymes of macrophages digest foreign
substances (intracellular digestion). In addition macrophages release lysozyme,
destroying bacteria; certain substances, which increase temperature,
interferon, inhibiting viruses multiplication, interleukin-1, stimulating DNA
replication in B- and T-lymphocytes, the factor, which stimulates the
production of antibodies by B-lymphocytes, the factor, stimulating the
differentiation of B- and T-lymphocytes, the factor, stimulating
chemotaxis of B- and
T-lymphocytes, and the cytotoxic
factor destroying malignant cells. Macrophages play an important role in the
immune mechanism. These cells are able to direct phagocytosis and release
oxidants. They are antigen-presenting cells.
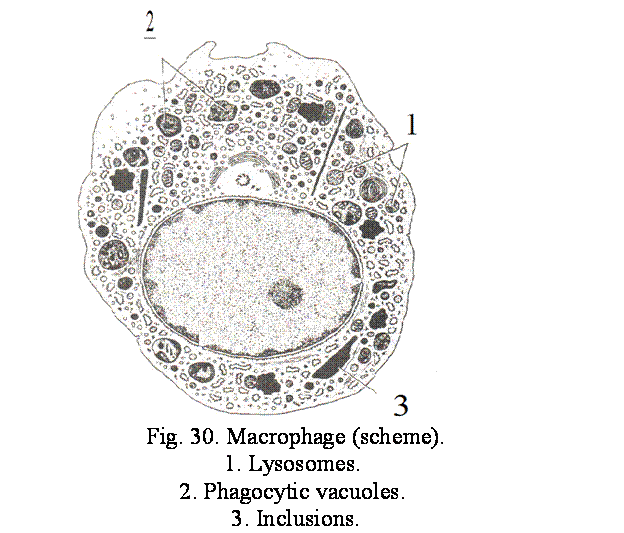
Macrophage system
It includes all cells of
the organism possessing 3 properties: 1) the ability to provide phagocytosis, 2)
their cell surface bears receptors to antigens, lymphocytes, immunoglobulins,
etc., and 3) all these cells are derived from blood stem cells or monocytes.
For example, 1) macrophages present in loose connective tissue, 2) stellate
macrophages of the liver, 3) macrophages of the lungs, 4) foreign body giant
cells, 5) osteoclasts of bone tissues, 6) macrophages present in peritoneal
fluids, 7) glial macrophages of the nervous system.
The founder of the
theory of the macrophage system is a
Mast cells (Basophilic granulocyte
of connective tissue)
Mast cells are derived
from blood stem cells. The cells are ovoid, round, elongated, etc. Their
nuclei, containing heterochromatin, are dense. The cell cytoplasm is slightly
basophilic; it contains large basophilic granules, which are about 1.2 mm in diameter (Fig. 31).
The contents of granules
is 1) crystalloid, lamellar, reticular, and mixed structures, 2) histamine, 3)
heparin, 4) serotonin, 5) chondroitin sulphate acid, and hyaluronic acid.
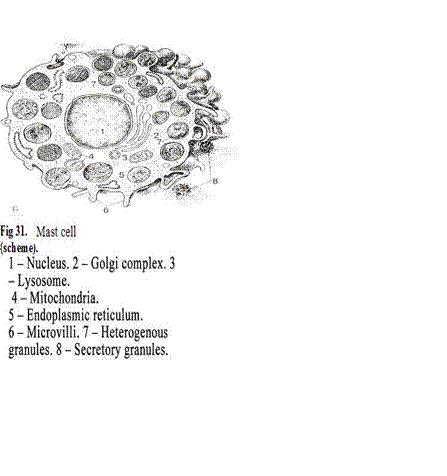
The cell cytoplasm
contains 1) lipase, 2) acid phosphatase, 3) alkaline phosphatase, 4) ATP, and
5) histidindecarboxylase. The latter is the marker of mast cells.
Mast cells release heparin, which relaxes the
permeability of the capillary wall and inflammation. When histamine is released
by mast cells the permeability of the capillary wall and ground substance of
connective tissue increases, inflammation is activated. Thus, mast cells
regulate local homeostasis (relax or activate inflammation) and bring about
allergic reaction.
The relationship between
mast cell and allergen leads to the release of histamine (degranulation)
because the former cell membrane bears receptors to immunoglobulins type E.
Mast cells are main cells, which bring about allergic reactions.
Plasma Cells
They are derived from
B-lymphocytes. The cells are ovoid or round. They are 8-9 mm in diameter. Their cytoplasm is
slightly basophilic. Near the nucleus there is a colourless region called the
small court where the Golgi complex and cytocenter are present. The nucleus of
plasma cells is round or ovoid and moved to the periphery by the small court.
The nucleus contains chromatin clamps, which makes it look like a cartwheel.
The cell cytoplasm contains prominent ER and ribosomes; the rest organelles are
moderately developed. The plasma cell function is the release of antibodies.
Adipocytes (Fat Cells)
These cells are located
in loose connective tissue. There are single fat cells or aggregations of the
cells (Fig. 32) in the tissue. Single adipocytes are round; the entire cell is
filled by fat (a fat droplet contains triglyceride, glycerin, fat acids,
phospholipids, and cholesterol). The cytoplasm and flattened nucleus of the
cell are pushed to the periphery by the droplet of fat. The cytoplasm of the
cell contains not prominent mitochondria, pinocytotic vesicles, and enzyme
glycerolkinasa. Adipocytes are sources of energy and water.
Adipocytes are derived from
undifferentiated cells. In the cytoplasm of the latter droplets of fat are
stored. Fat is absorbed from the intestine into blood as small droplets, which
are 1 mm in diameter (hilomicrones). The
droplets travel to the site where adipocytes or undifferentiated cells are
present. Here fat droplets are broken up by enzymes, released by endothelial
cells of capillaries to form glycerin and fat acids, which enter the
undifferentiated or fat cell. Here glycerin and fat acids are synthesized by
enzymes.
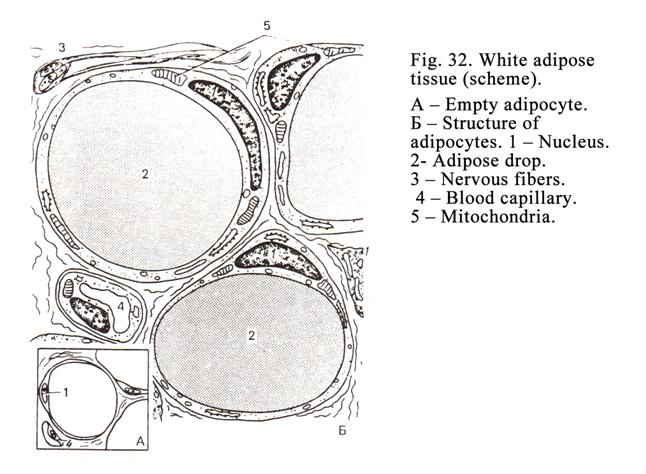
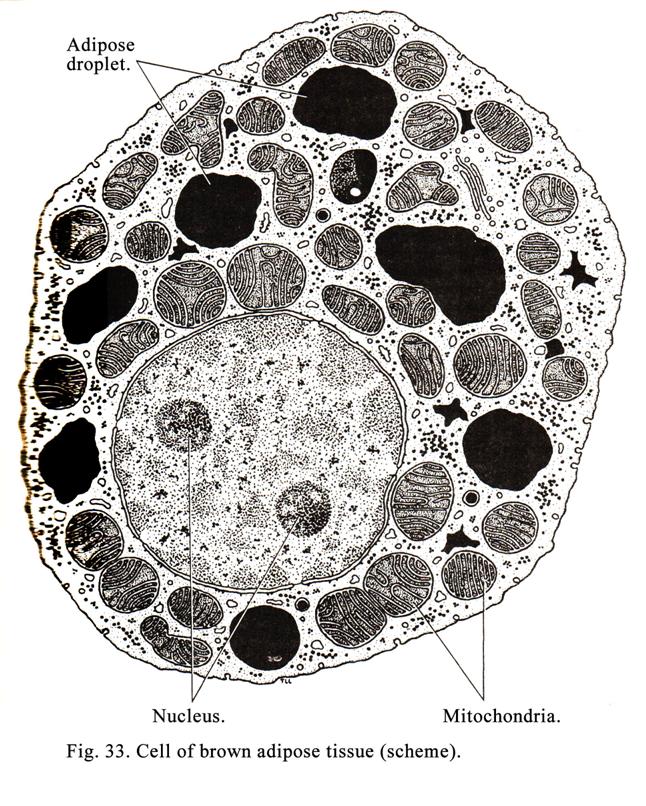
In the organism
adrenalin is released by suprarenal glands. Adrenalin is caught by the receptors
of adipocytes. Adrenalin stimulates adenylate cyclase; the latter stimulates
cyclic adenosine monophosphate (cAMP) synthesis, which stimulates lipase to
break up triglyceride to form glycerin and fat acid. The latter is discharged
from cells into the lumen of blood capillary vessels, where it joins protein to
form lipoprotein, which travels to the site where energy is needed. Insulin
stimulates the storage of fat in adipocytes and prevents lipid from leaving
these cells. If the quantity of insulin is not enough, adipocytes lose lipids,
so the
person is thin. In human body there are cells of brown adipos tissue
(Fig. 33).
Brown Adipose Tissue
In some part of the body
adpose tissue has a brown color. The cells in this type of tissue (Fig. 33)
differ from those in white that as follows.
1. They are smaller than in white
adipos tissue.
2. The fat in its cytoplasm occurs in form of
several small droplets.
3. The cytoplasm and nucleus of the
cell are not pushed to a cell membrane. The cytoplasm contains number
mitochondria.
Pigment Cells (Melanocytes)
They are present in
loose connective tissue, but they are not strictly cells of the connective
tissue because they are derived from the neural crest. They are dendritic
cells. In the light cell cytoplasm there are no prominent organelles but
melanin granules are seen well.
Undifferentiated Cells
The cells are spread
along blood vessels. They are spindle shaped. Their cytoplasm, containing
ribosomes and RNA, is slightly basophilic. These cells are divided by mitosis.
They undergo differentiation to form fibroblasts, myofibroblasts, adipocytes
and other cells.
Connective tissue contains many
leucocytes, entering from circulation and providing their own functions.
Pericytes
These dendritic cells are localized
in the blood capillary wall. Outgrowths of the cells contain contractile
filaments. When the filament contracts the capillary lumen constricts.
Intercellular Substance of Loose
Connective Tissue
It includes collagen fibres, elastic
fibres, reticular fibres, and ground substance.
Collagen
Fibres
These fibres consist of protein
called the collagen. Some collagen fibers constitute the bundle. Bundles may by
straight or wavy depending upon how they are stretched. They are 1-10 mm in diameter, have different
length. There are more than 15 types of collagens. Type I is found in bone,
reticular layer of the skin. Type II is found in hyaline cartilage, and
vitreous body of the eye. Type III is included in reticular fibers. Type IV is
in basal membranes and lens capsule. Type V formed, by smooth myocytes and
endothelial cells, forms the external cell skeleton. Other types of collagens
are less studied.
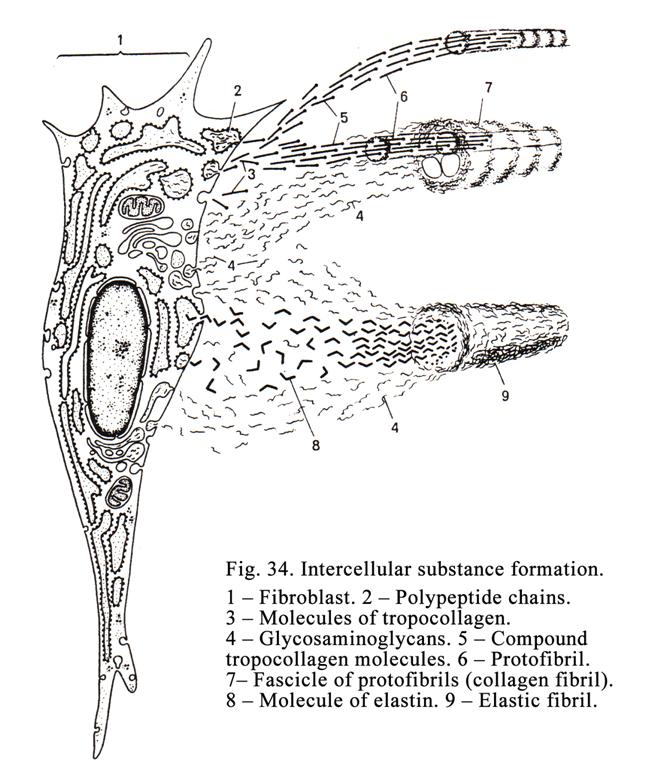
Formation
of Collagen Fibers
There are 4 stages of collagen
fibers formation (Fig. 34). The first
stage is called molecular (intracellular) stage, the second is called supra
molecular stage, the third is called fibrillar stage, and the fourth is called
fibrous stage.
The First Stage
At these stage molecules
of collagen (tropocollagens), synthesized by rough ER of fibroblast, are 280 nm
in length and 1.4 nm in diameter. Every tropocollagen consists of 3 chains of
amino acids arranged in a certain order. The molecules are released by the
entire surface of the cell membrane.
The Second Stage
During the second stage
tropocollagens are joined with each other to form the thinnest fibrils. 5-6
fibrils are joined by their side surfaces to form thicker fibrils, which are 10
nm in diameter.
The Third Stage
During the third stage
the thicker fibrils are joined by their side surface to form thick fibrils,
which are 50-100 mm in
diameter. Every fibril shows cross striations after every 64-67 nm.
The Fourth Stage
During the fourth stage
the thick fibrils are joined by their side surface to form collagen fibers or
bundles. The latter are 1-10 mm in
diameter.
The collagen fibers
provide the mechanical stability of connective tissue. For example, a collagen
thread may suspend
Elastic Fibers
These are thin and
straight joined which one another to form a wide net. Elastic fibres consist of
protein elastin. The fiber formation includes 4 phases. The first phase is
called molecular, the second phase is called supra molecular, the third phase
is called fibrillar, and the fourth phase is called fibrous (Fig. 35).
The Molecular Phase
During the phase small
globules, which are 2.8 nm in diameter, are synthesized by rough ER and
released by cells.
The Supra Molecular Phase
During the second phase
the globules are joined with one another to form chains, which are 3.5 nm in
diameter.
The Fibrillar Phase
During the third phase the
chains are covered by glycoproteins to form fibrils, which are 10 mm in diameter.
The Fibrous Phase
During the fibrous phase
the fibrils are joined with one another by their side surfaces to form
fascicles or tubules. They are called oxytalanic fibers. Then amorphous
substance fills the tubules to form elaunine fibers, containing 50% of the
amorphous substance. When the
amorphous substance constitutes 90%; the fibers are called mature elastic
fibers. Oxytalanic and elaunine fibers are not mature.
Elastic fibers can be
stretched (like a rubber band). Elastic fibers provide elastically of
connective tissue. They are less strong but more stretch than collagen fibers.
Reticular Fibers (Fig. 36Į)
They consist of protein
collagen type III. This protein is synthesized by fibroblasts. The reticular
fibers formation is similar to that of collagen fibers. In
reticular fibers there are transfers
of light and dark bands their width are 64-67 nm. Reticular fibres are less
strong but more stretchy than collagen fibres, they are also, more strong, but
less stretch than elastic fibers. Reticular fibers form a net.
Fundamental (Amorphous) Substance
This is semifluid
substance. The substance is formed partly by blood, partly by fibroblasts and
mast cells. Water, salts, albumins, and globulins leave blood vessels and enter
the intercellular space. Fibroblasts release glycosaminoglycans (chondroitin
sulphate, keratin sulphate, heparan sulphate, dermatan sulphate, and hyaluronic
acid) and glycoproteins. The quantity of hyaluronic acid influences the
consistence and permeability of fundamental substance. The most fluid substance
is near the blood and lymphatic vessels. A denser fundamental substance is deep
to the basal membrane.
The fundamental
substance provides the exchange between blood capillary vessels and parenchyma
cells. The formation of collagen, elastic, and reticular fibers occurs within
the fundamental substance. This substance provides the function of connective
tissue cells.
The intensity of
different substance exchange through the fundamental substance depends on its
(fundamental substance) permeability. It depends on the quantity of loose
water, hyaluronic acid, the activity of hyaluronidase, and sulphate
glycosaminoglycans and histamine concentration. The number of
glycosaminoglycans (hyaluronic acid) of fundamental substance provides
permeability inhibition. Hyaluronidase destroys the hyaluronic acid resulting
in permeability of fundamental substance increases. Histamine also increases
the fundamental substance permeability.
Basophilic granulocytes
and mast cells take part in permeability of fundamental substance regulation by
histamine and heparin release.
Eosinophil granulocytes also regulate the fundamental substance
permeability by histaminase release, which destroys histamine.
Hyaluronidase is within
bacteria and viruses. They release hyaluronidase so the permeability of
fundamental substance and capillary vessel walls increases that allow them to
enter the organism and bring about different diseases.
Dense Collagen (Fibrous) Connective
Tissue
It contains a very small
amount of cells and fundamental substance, but more fibers (mainly collagen
fibers). Dense connective tissue is classified into regular and irregular. For
example, irregular connective tissue is reticular layer of the skin; regular
connective tissue is the tendon.
Dense Regular Connective Tissues
These are tendons,
ligaments, aponeurosises of muscles, joint capsules, capsules of some organs,
sclera of the eye, albuginea tunics of the testis and ovary, dura mater,
periosteum, and perichondrium.
Tendons
They consist of orranged
in parallels collagen fibers. The fibers form the primary, secondary and
tertiary fascicles. The primary fascicles are separated from one another by
fibrocytes. Some primary fascicles constitute the secondary fascicle. The
latter is separated from each other by loose connective tissue called
endotenium. Some secondary fascicles constitute the tertiary fascicle of a
tendon. The tendon is surrounded by collagen connective tissue called
peritenium. Within the endotenium and peritenium blood and lymphatic vessels,
and nerves are present. The tendons join muscles with the skeleton.
Connective Tissue Lamina
Connective tissue
laminae are fascia, aponeurosis, perichondrium, etc. The lamina consists of
collagen fibers to form layers. Every layer consists of, arranged in parallels
collagen fibers to form lamina. Collagen fibers of every layer have their own
direction. Collagen fibers can leave their own layer and enter the adjoining
layer. Therefore separation of aponeurosis layers from each other is difficult.
Thus, connective tissue lamina is different from tendons because the former
criates layers; the latter form fascicles. Between the collagen fiber layers
there are fibrocytes and fibroblasts.
Ligaments
The structure of
ligaments is like that of the tendons but the arrangement of the fibres of the
former is not strongly parallel.
There exists the
ligament nuchae. It consists of elastic fibers. The structure of tunic albuginea,
perichondrium, periosteum, and dura mater is not similar to aponeurosis because
the direction of collagen fibers is not strictly parallel.
Dense Irregular Connective Tissue
Collagen fibers of skin
connective tissue reticular layer are arrangement not parallel to form the net
(reticulum). It is irregular connective tissue derived from mesoderm. The
reticular layer provides mechanical strength of the skin.
Connective Tissues with Special
Properties
These are adipose,
reticular, mucoid, and pigment tissues. The peculiarity of these tissues is the
predominance of definite line (differon) cells in them. For example, in adipose
tissue adipose cells are predominant; in pigment tissue pigment cells are
predominant.
Adipose Tissue
This is divided into white
adipose tissue and brown adipose tissue.
The White Adipose Tissue
It is located deep in the skin (subcutaneous
adipose tissue). Subcutaneous tissue is abundant in the skin of the abdomen,
femur, and buttocks, also in the large omentum, and small omentum. It consists
of adipose cells (adipocytes) filled with triglyceride to form large droplet
within the cell. Within connective tissue adipocytes form lobules surrounded by
layers of loose connective tissue, where blood and lymph capillary vessels and nervous
fibers are present. At prolonged hunger lipids are released from adipocytes so
the latter become star-shaped, the person becomes thin. When nutrition resumes
incorporation of glycogen appears in the cell, then drops of lipids appear,
which are combined to form a large drop. The latter pushes the nucleus and
cytoplasm to the periphery of the cell. On prepared slides drops of lipid of
cells are dissolved and cells become empty.
At the same places there
are lipids, which do not disappear during prolonged hunger. For example,
adipose tissue of the eye orbit, subcutaneous adipose layer of the palm and the
sole planta because this adipose tissue provides the mechanical cushion
function.
Brown Adipose Tissue
It is present in newborn
subcutaneous adipose tissue of the neck, between the shoulder blades, along the
vertebral column, and behind the sternum. The cells in this type of tissue
differ from those in white adipose tissue. They are smaller than in white
adipose tissue. These cells are polygonal, their cytoplasm and round nucleus
are not pushed to the periphery. Fat in the cytoplasm is in the form of several
small droplets. The cell cytoplasm contains numerous mitochondria, containing
the brown pigment.
Brown adipose tissue
possesses a high oxidative ability. Oxidation follows the production of much
warm energy. The latter warms newborns and small children. The action of
adrenalin and noradrenalin stimulates the splitting of cell lipids. Starvation
does not lead to a lot less of brown fat. Between the cells of brown adipose
tissue there are a number of capillary vessels.
Mucoid Connective Tissue
It is situated in the
umbilical cord. Mucoid tissue contains mucous cells (like fibroblasts), much
hyaluronic acid, fundamental substance, and some collagen fibers. Mucous cells
release much hyaluronic acid and a few collagen molecules. Hyaluronic acid
provides high firmness of the mucoid tissue providing the protection of
umbilical blood vessels, when the umbilical cord is compressed or bended.
Pigment Tissue
In white race people
there are a few pigment tissues. They are in the iris of the eye, around the
mammary gland nipple and anus, and skin of the scrotum. Most cells of this
tissue are melanocytes, which are derived from the nervous crest.
CHAPTER 6
SKELETAL (CARTILAGE AND BONE) TISSUES
Cartilage and bone
tissues are derived from skeletal mesenchyme. These are tissues of the internal
medium of the body. The tissues consist of cells and intercellular substance.
The latter is firm therefore provides support and mechanic functions.
Cartilage Tissues
They are classified into
hyaline, elastic, and fibrous (fibrocartilage) cartilage. The classification is
based on intercellular substance structure. The cartilage tissue contains 80%
of water, 10-15% of organic substances, and 5-7% non-organic substances.
Chondrohistogenesis (Cartilage
Formation)
It includes 3 stages: 1)
the formation of chondrogenic islands, 2) the formation of primary cartilage
tissue, and 3) differentiation of cartilage tissue (Fig. 37).
During the stage of
the formation of chondrogenic islands mesenchymal cells associate to form
islands. Island cells undergo multiplication and differentiation to form
chondroblasts, differentiating in chondrocytes.
During the second
stage the chondrocytes contain abundant rough ER, the Golgi complex, and
mitochondria. These cells produce type II collagen to form the intercellular
oxyphil substance.
During the third stage rough ER of chondrocytes is more developed than that at the second stage. Chondrocytes can synthesize not only collagen but also, chondroitin sulfates therefore, intercellular substances, around cells, are stained by basic dyes.
Mesenchymal cells,
surrounding the cartilage bud, form perichondrium around it. The perichondrium
includes 2 layers: 1) the external layer consisting of fibrous tissue and 2)
the internal layer consisting of loose connective tissue, containing precursors
of chondroblasts and chondrocytes.
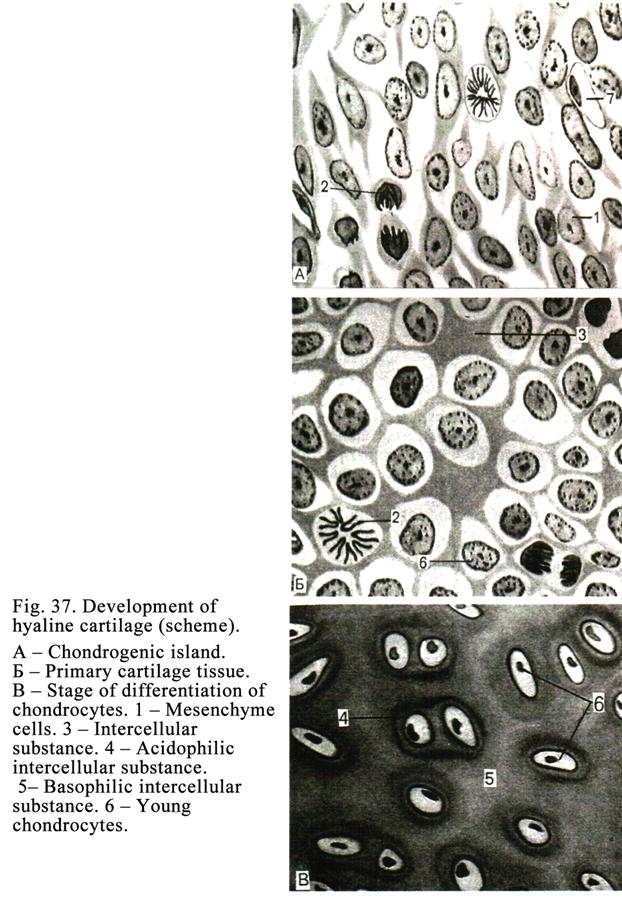 tist
tist
Appositional Cartilage Growth
The growth is possible owing
to the presence of chondroblasts in the deep layer of the perichondrium. These
cells are laid over the cartilage surface and undergo differentiation and
converting into chondrocytes. The latter form a new cartilage over the existing
cartilage.
Interstitial Cartilage growth
This kind of cartilage
growth is formed by multiplication of chondrocytes throughout its substance to
bring out the increase of the cartilage mass. Dividing cells release proteins
and chondroitin sulphates so the mass of cartilage increases.
Cartilage Cells
The cell chain includes
stem cells, semi-stem cells, chondroblasts, and chondrocytes.
Chondroblasts
They are present in deep
layers of perichondrium. They have many organelles (rough ER, the Golgi
complex, and mitochondria). The chondroblast functions are: 1) they secrete the
intercellular substance (fibrillar proteins), 2) they undergo differentiation
in chondrocytes, and 3) they undergo mitotic division.
Chondrocytes (Fig. 38)
They are localized within the cartilage lacunae. At first in the lacuna there is one chondrocyte, which divides to form 2, 4, 8 etc. cells. Therefore every lacuna contains many chondrocytes (chondrocyte aggregations).
The chondrocyte
aggregation cells are classified into 3 types (I, II, and III). Type I
chondrocytes can divide by mitosis. The cells contain the Golgi complex,
mitochondria, monosomes, large nucleus, and little few of cytoplasm. The cells
release only fibrillar proteins. They are present in young cartilages.
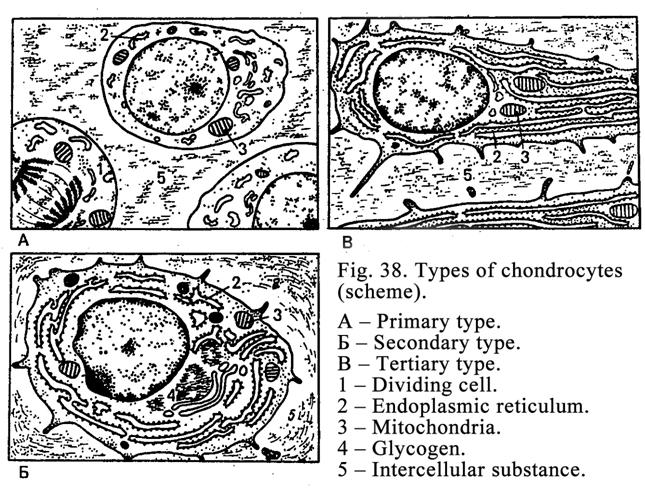
Type II chondrocytes are localized in mature cartilages.
The mass of the cytoplasm of these cells is more, than that in type I. This
type cells cannot divide. The cell cytoplasm contains abundant rough ER, they
release not only fibrillar proteins, but also chondroitin sulphates therefore
fundamental matrix around them is basophilic.
Type III the
chondrocytes is
localized in old cartilages. The cells lose the ability to synthesize
chondroitin sulphates and can only produce proteins. It leads to staining the
surrounding matrix by acid dyes. Thus, the fundamental matrix, surrounding
every aggregation of chondrocytes, includes 3 rings i.e. the innermost
fundamental matrix ring, surrounding the chondrocyte aggregation is stained by
acid dye (this fundamental matrix is released by type III cells). The intermediate ring of the fundamental
matrix surrounding chondrocyte aggregation is stained by basic dye (this matrix
is produced by type II cells). The outermost ring of the fundamental matrix
surrounding chondrocyte aggregation is stained by acid dye (this matrix is
produced by type I cells). Thus, these 3 rings demonstrate the process of
participation in producing fundamental matrix by type I cells, type II cells,
and type III cells.
Cartilage Fundamental Matrix
A fundamental matrix
contains organic substances (predominantly II type collagen), and non-collagen
proteins (glycosaminoglycans and proteoglycans). A big quantity of
proteoglycans provides high hydrophilic of fundamental matrix but latter
possesses firmness, and permeability. Through the fundamental matrix gases,
water molecules, ions and small protein molecules pass by diffusion from the
perichondrium. But macromolecules of protein cannot pass through the
fundamental matrix. Protein macromolecules are antigens. Because macromolecules
of proteins cannot pass through the fundamental matrix the immune reaction
against graft rejection cannot occur in transplant grafted from another
patient.
In the cartilage
fundamental matrix there are type II collagen fibres. The collagen fibres arrangement
depends on the stresses imposed on the cartilage. Blood vessels are absent
within the cartilage. Therefore the cartilage receives nutrition by diffusion
from perichondrium blood vessels.
The Hyaline Cartilage
It is of a
bluish-whitish colour, semitransparent, and fragile. The hyaline cartilage
(Fig. 39) is present in the costal joint with the sternum, in the trachea,
bronchi, larynx, and bone surface of the joint. The hyaline cartilage structure
depends on its location.
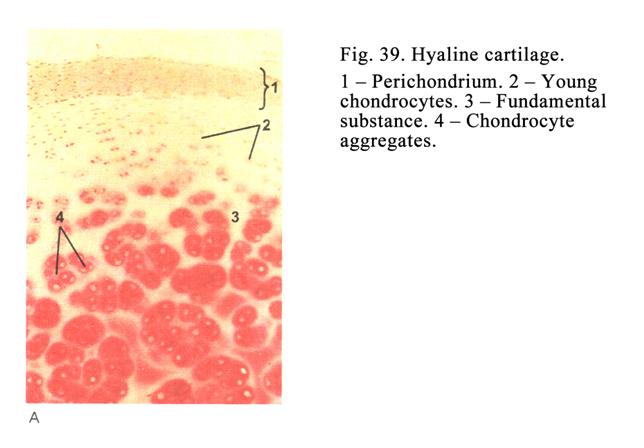
Costal Hyaline Cartilage
It is covered by the
perichondrium. Deep to the perichondrium there is a zone of young cartilage.
Here young spindle shaped chondrocytes are present. They are located in
cartilage lacunae. Young chondrocytes are able to release only fibrillar
protein. Therefore the fundamental matrix surrounding the cells is stained by
acid dyes. Deeper lined chondrocytes are round-shaped. Just deep to the zone of
young cartilage (superficial zone) the cells form chondrocyte aggregations.
They are able to synthesize not only proteins but also chondroitin sulphates
stained by basic dyes. Therefore the surrounding fundamental matrix is
basophilic. The innermost chondrocyte aggregations contain mature chondrocytes,
which are able to synthesize only proteins. Therefore the surrounding
fundamental matrix is stained by oxyphil.
The Hyaline Cartilage of the Join
Bone Surface
It is not covered by the
perichondrium. It has 3 zones (outermost, columnar, and innermost). The outermost
zone cells are small; spindle shaped, lying in the lacunae parallel to the
cartilage surface, and flattened. Chondrocytes of the columnar zone are larger,
more rounded, and rapidly dividing to form columns (cells stacked over one
another). The innermost zone is divided into 2 parts: non-calcified part and
calcified part covering the bone surface. The latter includes matrix vesicles
and blood vessels. Nutrition of this cartilage is provided by 1) synovial fluid
and 2) blood vessels of the deepest cartilage layer.
The
Elastic Cartilage
It is of a
whitish-yellowish colour, localized in the auricle, external acoustic meatus
wall, arytenoid and corniculate cartilages of the larynx, epiglottis, bronchi
of middle caliber. Elastic cartilage differs from hyaline in that the former
contains not only collagen fibers but also elastic fibers running in various
directions and entering the perichondrium. These fibres are stained by orcein
in brown colour. The elastic cartilage contains less chondroitin sulphates, lipids,
and glycogen, then hyaline that; never undergoes calcification.
The
Fibrocartilage
It is located between
the vertebral disks, pubic union, of tendon joined to the hyaline cartilage,
and others sites. This cartilage is joined to the tendon and other side to the
hyaline cartilage. At the site, where tendon is present, fascicles of collagen
fibers have parallel direction; between the fascicles there are fibrocytes. At
the site, where fibrocartilage is situated, collagen fibres arrange parallels
with one another but they are surrounded by chondrocytes present in the
lacunae. At the site where the hyaline cartilage is present, the latter has the
usual structure.
Age Related Changes in Cartilage
Cartilages are subjected
to alteration mostly in old people. At the same time the amount of
perichondrium chondroblasts decreases. Chondrocyte division becomes rare. Rough
ER, the Golgi complex, and mitochondria reduce. Chondrocytes lose the ability
to synthesize glycosaminoglycans and proteoglycans. This leads to the decrease
of hydrophilic ability, permeability, and the nutrition of cartilage. Therefore
the cartilage fundamental matrix undergoes calcification, blood vessels enter
the cartilage, and bone is formed within the cartilage.
THE BONE
The fundamental (ground)
substance of the bone is firm therefore the bone functions are as follows: 1)
supporting and mechanical functions, 2) accumulation of salts. The bone
contains about 70% of salts, the rest are water is and organic substances.
Collagen type I is predominant in the bone. It also contains other proteins,
citric acid, chondroitin sulphate acids, and glue substance.
The bone is classified
into 1) reticulofibrous bone tissue and 2) lamella bone tissue.
Reticulofibrous Bone Tissue
It consists of bunches
of collagen fibers oriented in different directions. Within the bone there are
osteocytes lodged in lacunae. After birth this bone is present between the
skull bones and places where tendons are joined to the bone.
Lamella Bone Tissue
It consists of collagen
fibers arranged parallel with each other to form lamellae.
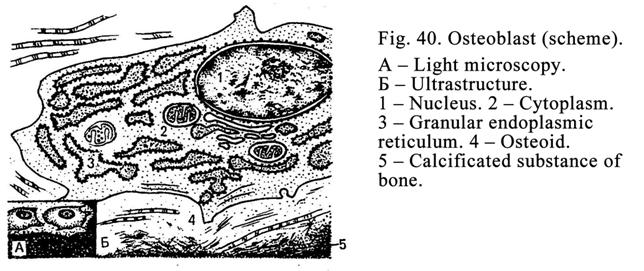
Bone Cells
They include 1)
osteoblasts, 2) osteocytes, and 3) osteoclasts (macrophages). The first are
stem osteogenicus cells undergoing differentiation to form osteoblast and
osteogenicus cells of the bone marrow.
Osteoblasts and Osteocytes
Osteoblasts (Fig. 40) are in the periosteum and are
placed where regeneration of the bone occurs. They have elongated form (the
length is 15-20mm),
oval nucleus,
oxyphil or basophilic cytoplasm,
abundant rough ER, the Golgi complex, and active alkaline phosphatase, but they
cannot undergo division by mitosis.
Functions of
osteoblasts: 1)
they release the glue substance, type I collagen (from which collagen fibers
are formed), chondroitin sulphates, and citric acid, and 2) these cells take
part in the process of bone calcification by alkaline phosphatase.
Osteocytes (Fig. 41) are branching cells placed in bone
lacunae. Processes of osteocytes occupy the canaliculiarising from the lacunae.
Usual organelles of the cells are developed slightly, their
nuclei contain heterochromatin, and nucleoli are absent. The functional
activity of osteocytes is less than that of osteoblasts. The functional
significance of osteocytes is in that they keep homeostasis of bone tissue.
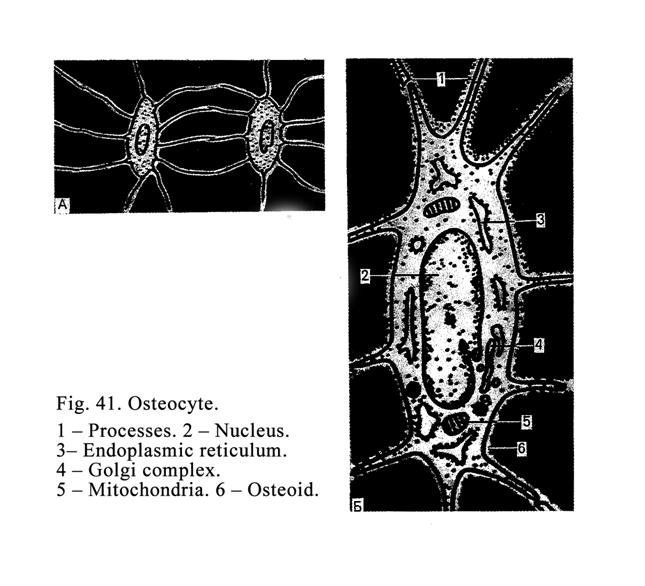
Osteoclasts
Osteoclasts (Fig. 42) are derived from blood stem cell (they undergo in line of hemopoietic
cells in the bone marrow) then monocytes, leaving the blood stream, entering the
bone tissue, and converting in osteoclasts. The size of the osteoclast may be
about 90 mm; they have different forms (oval,
elongated, irregular). The osteoclast surface, applied to bone, has two zones:
1) central or ruffed zone and 2) peripheral (zone of tight contact) zone. The
latter has a few organelles this zone is dense. The zone is very important
because osteoclast is joined with the bone by the zone to form airtight space
for the central zone.
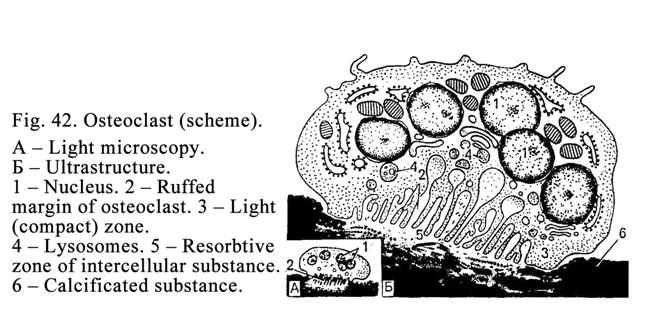
In the ruffled zone of
osteoclasts there are outgrowths bearing enzymes. Above the ruffled zone there
are various vacuoles, abundant lysosomes, containing proteolytic enzymes, and
mitochondria. The osteoclast contains 3-30 nuclei. These cells are located in
perivascular spaces of vessels, passing in bone canals, and the sites where
bone regeneration takes place.
The osteoclast lysosomal
enzymes break up the bone intracellular substance. The cells release carbon
dioxide synthesizing with water to form the carbonic acid so acidic environment
is formed, which activates the osteoclast function.
Bone Formation (Osteogenesis)
There are two manner of
bone formation: 1) membranous bone formation and 2) cartilage osteogenesis.
Membranous bone formation is the development of the bone
from mesenchyme directly. Before the
beginning of cartilage osteogenesis the pattern of hyaline cartilage similar to
the bone is formed, after it a typical long bone is developed instead of the
cartilage pattern.
Membranous Bone Formation
It includes 4 stages: 1)
osteogenicus islets formation, 2) bone formation, which is not mineralized
(osteoid), 3) calcification and reticulofibrous bone formation, and 4)
formation of the lamella membranous bone instead of the reticulofibrous bone.
At the first stage mesenchymal cells gather to osteogenicus
islets. The islet cells undergo differentiation to convert in osteoblasts.
These contain abundant rough ER, the Golgi complex, mitochondria, and alkaline
phosphatase.
At the second stage osteoblasts release type I collagen
and the glue substance to form the intercellular substance i.e., elongated
cords of the fundamental matrix (osteoid), which is not calcified. On the
surface of the cords there are osteoblasts, which form new fundamental matrix.
Therefore the cords become longer and thicker. In the process of cord formation
some osteoblasts are concluded by the fundamental matrix and undergo conversion
in osteocytes present in the lacunae. Other mesenchymal cells undergo
differentiation to convert in new osteoblasts, which continue the fundamental
matrix of cord formation. The formed cords join with each other to form a
network consisting of osteoid.
At the third stage the alkaline phosphatase releases
from osteoblasts. The phosphatase splits glycerol-phosphates into carbohydrates
and phosphate acid. The latter joins with calcium to form calcium phosphate,
which lines the fundamental matrix.
At the fourth stage reformatting calcium phosphate
undergoes conversion in crystals of hydroxyapatites joining with each other and
to collagen fibers by the glue substance.
Matrix vesicles take
part in calcification of the fundamental matrix. The vesicles are formed from
protrusion of osteoblast cell membrane at finally the protrusions separate from
the cells. They are 1 mm in
diameter and contain glycogen and alkaline phosphatase. In vesicles molecules
of calcium are deposited. The calcification by the matrix vesicles is divided
into 2 steps: 1) a crystal forms within matrix vesicle, 2) the vesicle membrane
undergoes rupture as a result the crystal leaves the vesicle and joins with
collagen fibres by the glue substance.
As a result of
calcification the reticulofibrous bone is formed. Around this bone the
periosteum is formed from surrounding mesenchymal cells. The periosteum
consists of 2 layers: 1) the internal osteogenic layer and 2) the external
fibrous layer.
At the fourth stage
blood vessels along with osteoblasts and mesenchymal cells enter the
reticulofibrous bone from periosteum. Across the wall of blood vessels
monocytes travel into the reticulofibrous bone, undergo differentiation, and
convert in osteoclasts. The latter remove the reticulofibrous bone to form
various shape holes. In center of every
hole there is a blood vessel. At the walls of hole osteoblasts lay down the bone
lamellae to form the membranous bone (lamella bone tissue). The osteoblasts
conclude themselves by bone and convert in osteocytes situated in bone lacunae.
Same concentric lamellae constitute the osteon or the Haversian system. The
osteons interlace to form the membranous bone. Between the interlacing osteons
there are mesenchymal and osteogenic cells, layers of connective tissue, and
blood vessels. Thus, the reticulofibrous bone is replaced by lamellar bone.
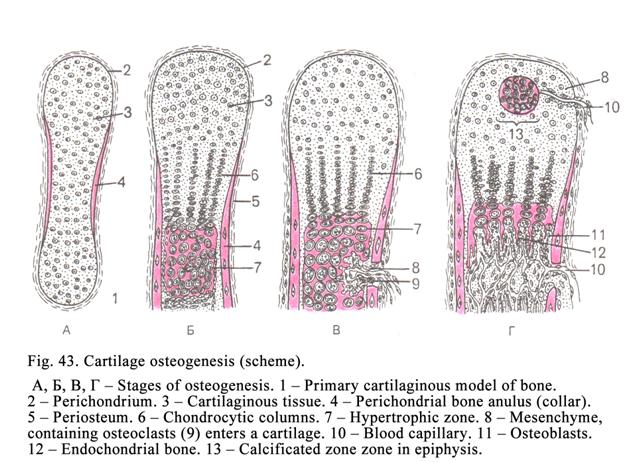
The osteoblasts, lining
the internal layer of periosteum form the external circumferential lamellae so
the entire bone is surrounded by the same lamellae. After forming of the bone
osteoclasts destroy the existing osteons, at the same time osteoblasts form new
osteons. The rebuilding of bone lasts life-long.
Cartilage Bone Formation
First the hyaline
cartilage pattern is formed (Fig. 43). Its form is similar to that of future
bone. The pattern is surrounded by perichondrium and has a diaphysis and two
epiphyses. Endochondrial ossification starts in the central part of the
cartilaginous pattern diaphysis. First osteoblasts present in perichondrium
leave the latter and form the reticulofibrous bone surrounding the diaphysis to
form the perichondrial bone anulus. The hyaline cartilage, surrounded by the
anulus, undergoes degeneration and calcification. The chondrocytes of the
diaphysis swell (giving the cells a vacuolated appearance), their nuclei are
pyknotic. At the same time the perichondrium is converted into the periosteum.
Bone buds, consisting of blood vessels, mesenchymal cells, osteoblasts, and
osteoclasts, enter the calcified cartilage from the periosteum through the
perichondrial bone anulus. The osteoclasts of the buds remove the calcified
cartilage to form holes of different shapes. On wall of the holes osteoblast
put down the bone substance called endochondrial bone. The peculiarity of this
bone is that within the bone there is the calcified cartilage. This process is
called endochondrial ossification. The endochondrial bone again undergoes destruction
by osteoclasts to form the marrow cavity. Mesenchymal cells, entering the
marrow cavity, form the endosteum (similar to the periosteum) lining the bone
marrow cavity wall.
The mesenchymal cells
form a bone marrow reticular stroma in the marrow cavity. Stem cells enter the
reticular stroma. At the same time the blood formation starts.
The reticulofibrous bone
of the perichondrial anulus is also removed by osteoclasts to form longitudinal
cavities called primary Haversian canals. Within every canal there is a blood
vessel. Osteoblasts lay down circular bone lamellae on the canal wall to form
osteons directed along the longitudinal axis of long bones. At the same time
osteoblasts leave the periosteum and form circumferential bone lamellae surrounding
the external surface of bone diaphysis.
At the same time
osteoblasts, located in the endosteum, lay down concentric bone lamellae on the
lumen surface of the marrow cavity as a result 3 layers of diaphysis are formed:
1) the external circumferential lamella layer, 2) the osteon layer, and 3) the
internal circumferential lamella layer lining the bone marrow cavity.
Epiphysis formation. When perichondrial bone anulus is
formed the cartilage epiphysis grows. The cartilage epiphysis (epiphiseal
cartilage) is divided into 3 zones: 1) reserve zone (resting zone), 2)
proliferative zone (chondrocyte column zone) where chondrocytes are divided by
mitosis and stacked over each other to form columns, and 3) hypertrophic zone
where chondrocytes assume the vesicular appearance and the surrounding matrix
is calcified. Diaphysis osteoclasts (osteoclasts present in diaphysis) remove
the hypertrophy zone of epiphiseal cartilage to form cavities. On the cavity
walls osteoblasts depose bone substance. Thus, the diaphysis bone replaces the
hypertrophic epiphysis zone. It allows the growth of diaphysis in length. The
cartilage epiphysis increases therefore nutrition of cartilage is disturbed. It
leads to the calcification of the central epiphysis part. Therefore blood
vessels, osteoclasts, and osteoblasts enter the epiphiseal cartilage to form a
bone within it. But between the bone epiphysis and bone diaphysis the cartilage
layer, called the epiphiseal growth plate, is present. The plate allows the
growth of the long bone in length in men by
The epiphiseal growth
plate includes 3 zones: 1) the resting (adjusting) zone with irregularly
arranged cells, 2) the proliferative zone, where the chondrocytes undergo multiplication
to form vertical parallel columns, and 3) the ossification zone. The zone is
removed constantly and replaced by a bone. Thus, in epiphiseal growth plate
occur both proliferative and removing processes. Chondrocytes provide the
proliferative process; the chondoclasts provide the removing process. When
proliferative process predominates, the bone grows in length, when the removing
process predominates the growth stops.
The thickness of bone
growth is provided by osteoblasts present in the periosteum and the endosteum.
The osteoblasts provide some external circumferential and some internal
circumferential lamellae as a result the bone thickness increases.
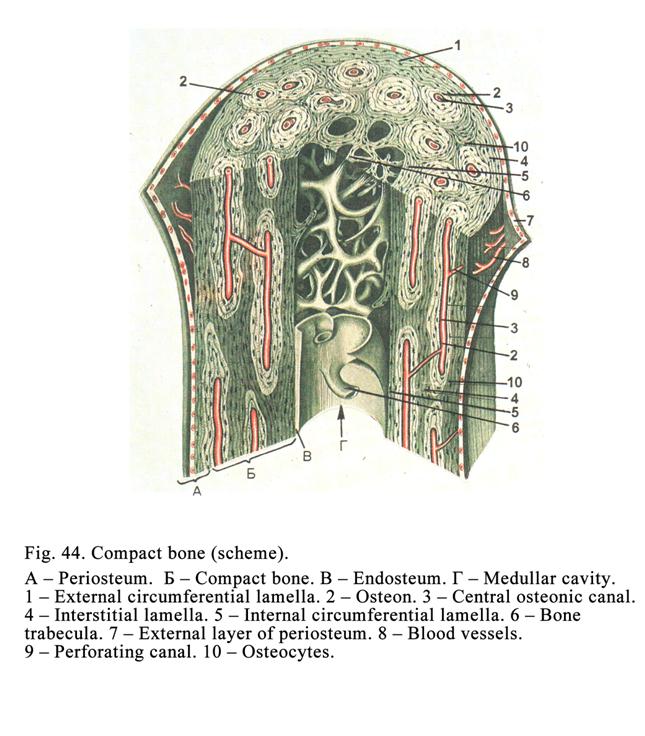
The lamellar bone is
divided into 1) compact bone substance (the long bone diaphysis) and 2) spongy
bone substance (the log bone epiphysis and membranous bone). The lamella is a
structural and functional unit of the spongy bones; the osteon is that unit of
the compact bone.
Long Bone Diaphysis (Compact Bone)
Structure
The external surface of bone
diaphysis is covered by the periosteum, the internal that is lined by the
endosteum. Between the periosteum and endosteum the compact bone (Fig. 44)
lies. The latter consists of three layers: 1) the external circumferential
lamellae, 2) the layer of osteons and interstitial lamellae, and 3) the
internal circumferential lamellae.
The External circumferential
lamellae
It consists of about ten
lamellae. Every lamella is 4-15 mm thick. Collagen fibers of every lamella are
parallel to each other. Collagen fibers of a lamella are in angle to the fibers
of the adjoining lamella. From the periosteum collagen (perforating or fibers
of Sharpey) fibers and perforating canals (canals of Volkmann) enter the
external circumferential lamella layer. Trough the canals nutrient arteries
pass. In every lamella and between them there are branching osteocytes located
in bone lacunae. The external circumferential lamellae are incomplete cylinders
surrounding the entire diaphysis but they partly put on one another covering the
entire diaphysis.
Layer of Osteons and Interstitial
Lamellae
The second layer (layer
of osteons) consists of osteons (of the Haversian systems) and interstitial
lamellae. Every osteon consists of some (usually 6) circular lamellae.
In the osteon center there is a canal through which blood vessels pass. The
osteon canals join with each other by perforating canals. Through these canals
the osteon blood vessels join with each other. Blood drains into the marrow
cavity through these blood vessels. Osteons are connected with each other by
cement lines.
Interstitial lamellae,
located between osteons, are remnants of broken down primary osteons. In the
interstitial and osteon lamellae and between them there are branching
osteocytes located in the lacunae. The lacunae communicate with one other by
fine bone canaliculi. Through the canaliculi the nutrient fluid circulates,
which feeds the bone. Therefore they are called the nutrient bone canaliculi.
Internal Circumferential Lamellae
They are similar to
external circumferential lamellae. They separate the osteon layer from the bone
marrow cavity.
Spongy Bone Tissue
This is also lamella
bone. It consists of osteons, but the latter interlace to form a somewhat
altered shape. The structural unit of the spongy bone is the bone lamella.
Between spongy bone cords (osteons) there are spaces filed with the bone
marrow.
The bone is provided by
nutrient trough periosteum blood vessels, central canal osteon blood vessels,
perforating canal blood vessels, and endosteum blood vessels. From perivascular
spaces nutrients enter the bone canaliculi, which supply the bone tissue.
Rebuilding the Bone Tissue &
Internal and External Factors Influencing the Rebuilding Process
Throughout the entire
life the bone tissue is reformed by osteoclasts and osteoblasts. Osteoclasts
remove the existing osteons to form cavities at the same time osteoblasts make
new lamellae to form osteons surrounding blood vessels.
Exocrine Factors Influencing the
Bone Rebuilding
Mechanical actions influence the bone tissue
reformation because they stimulate the function of osteoblast to form new
osteons as a result the bone becomes dense and strong. If the mechanical
actions are weak, osteoclasts are activated the latter remove the bone tissue
therefore it becomes weak and thin (for example in a weightless condition).
Electricity influences the bone structure. The
concave and convex surfaces of bone lamella have different electric potential.
The positive potential stimulates the activity of osteoclasts therefore the
bone is destroyed here, the negative potential stimulates the activity of
osteoblasts therefore bone is formed. Surgeons use this fact. If it is
necessary to form an additional bone tissue, surgeons make a negative electric
potential in this place.
Vitamins C, D, and A strongly influence bone rebuilding.
Vitamin C activates the osteoblast functions i.e., the cells intensively form
collagen fibres and take part in ossification of bone by discharging alkaline
phosphatase. If vitamin C is not enough, the bone tissue becomes mild.
Vitamin D influences the
bone calcium and blood calcium level. Vitamin D deficiency causes the bone
osteomalacia in adults and rickets in children.
Increased intake of
vitamin A stimulates the activity of osteoclasts, which remove the bone tissue.
Internal Factors (hormones)
Influencing Bone Reformation
The thyroxin deficiency
is similar to vitamin C activity i.e., the activity of osteoblasts decreases
that leads to the disturbance of collagen fibres synthesis and ossification.
The high blood livel of
thyro-calcitonin causes bone ossification activation.
A high level of the
blood parathyroid hormone causes activation of the osteoclasts, having the
receptors to the hormone (they are target-cells to this hormone) as a result
calcium is removed from the bone. The latter becomes weak.
The somatotropin lack
(produced by hypophysis) leads to the disturbance of the long bone growths.
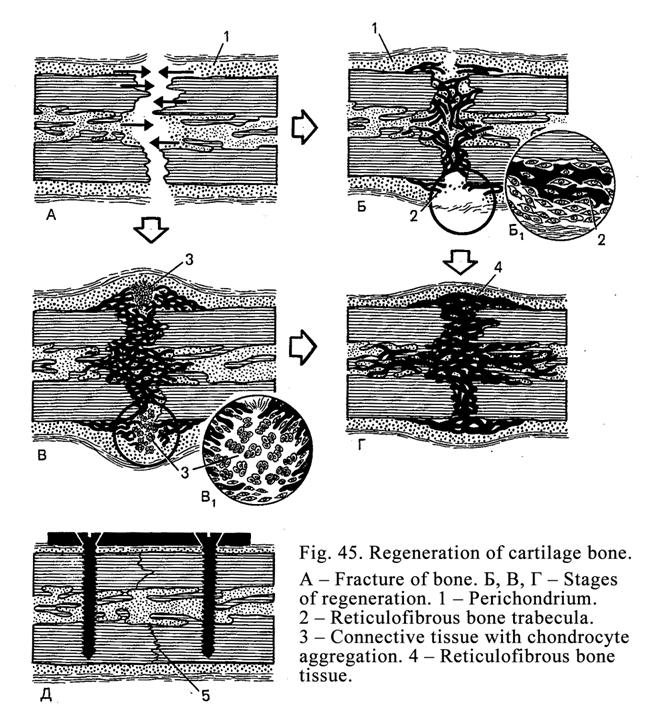
A low level of sex
hormones before puberty inhibits removing of the epiphiseal cartilage therefore
young man can grow up to 30 year old. In contrast to it high level of sex
hormones causes early removing of the epiphiseal cartilage as a result the
growth of the long bone stops.
The lack the sex
hormones after menopause leads to the disturbance of the bone structure
therefore the woman must be treated by sex hormones.
Bone Regeneration (Fig. 45)
After the bone fracture
2 or more broken bones may be formed (compound fracture). Osteoclasts migrate
to the ends of the broken bone and engulf necrotic particles of the bone (clean
up the ends of the broken bone). After it osteoblasts form non-calcified bone
connecting the ends of the broken bone. Then the non- calcified bone undergoes
calcification as a result it converts to the hard bone.
Knitting of the broken bone
ends may be activated if the patient is treated with vitamin A stimulating
osteoclasts at the first day. After it the patient must be treated with vitamin
C stimulating osteoblasts depositing the bone substance. Vitamin C deficiency
leads to the formation of spurious joints.
Bone Junctions
The junctions of bones
are divided into: 1) continuous junctions and 2) discrete junctions.
Continuous Junctions
a)
Bone
junctions by dense connective tissue are parietal suture of the skull, connective tissue membrane between the
fibular and the radius.
b)
Bone
junctions by cartilage are cartilage discs between the vertebral vertebrae.
c)
Junctions without
connective tissue are junctions
of the pelvic bones
Discrete Junctions (Joints)
They consist of the
articulated ends of bones covered by the hyaline cartilage and encircled
(surrounded) by the joint capsule. The capsule consists of two layers: 1) the
external layer and 2) the internal layer.
The external layer consists of dense regular
connective tissue. The internal (synovial) layer consists of 1) lamella of deep
collagen and elastic fibers, 2) lamella of superficial collagen and elastic
fibers, and 3) covering lamella adjoining the lamella of superficial collagen
and elastic fibers. The covering lamella consists of 3 types of the synovial
cells: 1) macrophages, 2) synovial fibroblasts, and 3) intermediate cells.
CHAPTER 7
THE MUSCLE TISSUE
The muscle tissue is
classified into smooth and striated muscle tissues. The striated muscle tissues
are further divided into skeletal and cardiac tissues. According to the origin
muscle tissues are divided into 5 types: 1) mesenchymal (smooth muscle tissue),
2) epidermal (smooth muscle tissue), 3) nervous origin muscle tissue (smooth
muscle tissue), 4) celomic (cardiac muscle tissue), and 5) somatic (skeletal
muscle tissue).
Smooth Muscle Tissue
Mesenchymal smooth
muscle tissue is derived from mesenchymal cells and
present in the walls of hollow viscera (the stomach, the intestine, the urinary
bladder, etc.), in the walls of several structures that are in the form of
narrow tubes (arteries, veins, bronchi), in the non-hollow organs (the eye
ciliary body). Mesenchymal cells (origin of the smooth muscle cells) lose
processes. In the cell cytoplasm there are the Golgi complex, mitochondria,
rough ER, and myofilaments. At the same time on the rough ER surface collagen
of the fifth type is synthesized, which is used in forming the basal lamina.
With further differentiation organelles of cells partly undergo atrophy, the
synthesis of collagen molecules is decreased, but the synthesis of retractive
proteins of myofilaments is increased.
Mesenchymal Smooth Muscle Tissue
Structure
The smooth muscle is made up of long
spindle-shaped cells (myocytes) having the broad central part and tapering
ends. The smooth muscle cell (Fig.46) length is 20-500 mm; every smooth muscle cell is
covered by the cell membrane, the latter is covered by the basal membrane.
Myocytes are closely contacted with each other. Between the cells there are
communication junctions (gap junctions). In the place of the basal membrane,
where the gap junctions are located the orifices are. Through the orifices cell
membranes of adjacent myocytes approach each other at the distance of 2-3 nm to
form gap junctions. The smooth muscle cell surface is covered by the external
cytoskeleton consisting of collagen type 5. The muscle cell cytoplasm is
oxyphil. The cell cytoplasm contains weakly developed rough ER, the Golgi
complex, the cytocenter, and lysosomes. These organelles are located near the
poles of the nucleus. The cells contain abundant mitochondria. The nucleus of
the smooth muscle cell is spindle (rod) shaped.
Myofilaments of myocytes
(retractive apparatus of cells) are developed well. Myofilaments are divided
into 3 types: 1) thin filaments consisting of retractive protein called actin,
2) thick filaments consisting of retractive protein called myosin, which is
formed after traveling of nervous impulse to the cell, and 3) intermediate
filaments consisting of special proteins. In smooth muscle cells transverse
striations are absent because their filaments are arranged irregular. Actin
(thin) filaments are joined with each other and cell membrane by electron dense
bodies consisting of special proteins.
Actin filaments
predominantly are arranged along the cell length, but it may be the oblique
direction. Myosin filaments are also predominantly directed longitudinally.
Both actin and myosin filaments are arranged so that the ends of the former and
the latter extend between each other.
The filaments (actin and
myosin) produce the contraction of smooth muscle cells. The contraction process
occurs as follows. After myocytes receives a nerve impulse the pinocytotic
vesicles, containing calcium ions, move to the filaments. Calcium ions produce
moving ends of the actin filaments between those of the myosin filaments. The
peripheral ends of the actin filaments attach to the cell membrane of the
smooth muscle cell. The moving actin filaments pull the cell membrane as a
result the smooth muscle cell contracts.
Myocytes function: 1)
they are capable of a prolonged contraction and 2) they synthesize (by their
rough ER) and release type V collagen, elastin, and proteoglycans.
Smooth Muscle Tissue Regeneration
The regeneration of
these sells is provided: 1) by division of myocytes and 2) by conversion of
myofibroblasts into myocytes.
Organization of Myocytes and
Connective Tissue in Smooth Muscle
Within the wall of the
hollow organs the smooth muscle forms fascicles, surrounded by delicate sheath
of connective tissue, called perimysium. The entire layer of the smooth muscle
is surrounded by stronger sheath of connective tissue called the epimysium.
Within the perimysium and epimysium there are blood and lymphatic vessels, and
nerve fibers.
The smooth muscle is
supplied by autonomic nervous system therefore the muscle contraction is
involuntary. Both sympathetic and parasympathetic nerves end on the muscle
tissue by motor ending. When a nerve impulse reaches the nerve ending, the
transmitters release from it. The transmitters (noradrenalin, adrenalin or
acetyl choline) travel through connective tissue to muscle cells providing
their contraction or relaxation. Sensitive nerve fibers are also present in
smooth muscle tissue.
Epidermal Smooth Muscle Tissue
It is derived from
ectoderm and represented by myoepithelial cells. These, containing retractive
myofilaments, are star shaped. The sells are located in salivary, sweat,
mammary, and lacrimal glands between the cellular base and the basal membrane.
When myoepithelial cell contracts, the cellular base of glandular cells is
squeezed as a result secretion releases from the glandular cell.
Nervous Origin Smooth Muscle Tissue
This is developed from
ocular bud arising from the nervous tube. The muscle tissue only forms 2
muscles located in the iris: 1) sphincter pupilla muscle and 2) dilator pupilla
muscle. The muscle is believed to be developed from glial cells.
Skeletal Muscle Tissue
Skeletal muscle tissue
is connected with the skeleton.
Skeletal Muscle Development
The muscle is derived from mesoderm.
Mesoderm cells undergo differentiation in 2 directions: 1) one part of the
cells converts in satellite cells, 2) the second part converts in myoblasts.
During maturation myoblasts collect to form myotubes, their nuclei move to the
peripheral part, but their myofibrils move to the center. Satellite cells enter
the myotubes. After it the myotubes are called the muscle fibers (striated
muscle cells).
Muscle Fiber Structure
The muscle fiber (Fig.
47) includes the satellite cells. The fiber is 5-
The nuclei of the muscle
fiber line sub plasmolemma. Every muscle fiber includes some thousands of
nuclei. The nuclei are elongated in shape. They lose the ability to divide by
mitosis. The cytoplasm of the muscle fibers is called the sarcoplasm. The
sarcoplasm contains myoglobin, glycogen, and lipids. The Golgi complex, rough
ER, and lysosomes are located at poles of the nuclei. These organelles are
developed weakly while mitochondria and smooth ER (sarcoplasmic reticulum) are
developed well.
Muscle fibers contain a
number of myofibrils constituting the retractive apparatus. Myofibrils show
transverse striations because the fibrils are arranged regularly. Myofibrils
consist of 2 types of myofilaments: 1) thin actin filaments, including actin,
troponin, and tropomyosin, and 2) thick myosin filaments consisting of myosin
protein. Actin filaments are directed along the myofibril their ends are arranged
in one level but that of the myosin filament is a little extended. One end of
myosin filament is surrounded by 6 ends of actin filaments. In the muscle fiber
there is the cytoskeleton represented by intermediate filaments, the Z-line,
the M-line, and sarcolemma. The cytoskeleton provides regular arrangement of
myofibril structures (actin and myosin filaments, etc.). The myofibril part,
consisting only of actin filaments, is isotropic band (I band). The isotropic
band center is divided by the Z-line (Z-band) into 2 equal parts. The Z-band
thickness is about 100 nm. It consists of a-actinin. Actin filaments are attached to
Z-band (zone of attachment of actin filaments).
Myosin filaments are
also arranged in stringent order; their ends are at the same level. Myosin
filaments with extending ends of actin filaments constitute the anisotropic
band (A-band) processing birefringence properties. The A-band is divided by the
M-band (M-line) consisting of special protein.
The A-band center is
traversed by a lighter band called the H-band limited by ends of actin
filaments. Therefore if the distance between the ends of actin filaments
becomes shorter, the H-band is narrower.
The Sarcomere (Myomere)
The myofibril part
situated between two Z-bands is called the sarcomere. The sarcomere (Fig. 48)
formula is ½ half of I-band+A-band+1/2 half of
I-band.
Every myofibril is
surrounded by prominent mitochondria and smooth endoplasmic reticulum
(sarcoplasmic reticulum). The sarcoplasmic reticulum (Fig. 49) forms a system
of tubules opposite every I-band and A-band. The system consists of
longitudinal and transverse tubules. The latter are called the terminal
cisterns. The longitudinal tubules are joined with transverse tubules to form
sarcoplasmic network in every system. The sarcoplasmic reticulum functions: 1)
transport of some substances, 2) lipids and carbohydrates synthesis, and 3) Ca++
ions accumulation.
Transverse (T)
tubules are tubular
extensions of sarcolemma into the sarcoplasm between 2 adjoining terminal
cisterns.
Triads (Fig. 49 II 8)
These include 1) the
T-tubule and 2) 2 adjoining terminal cisterns. The triad function: when
myofibril is relaxed, C++ ions accumulate in the terminal cisterns,
when nerve impulse reach the T-tubule,
Ca++ ions leave the terminal cisterns and move to actin filaments.
The myofibril contraction cannot occur without Ca++ ions. In the
relaxed condition actin filaments are blocked by tropomyosin therefore molecule
heads of myosin filaments cannot join with actin filaments. Ions of Ca++
remove tropomyosin from actin filaments to allow interaction between these
filaments and the contraction of myofibril begins.
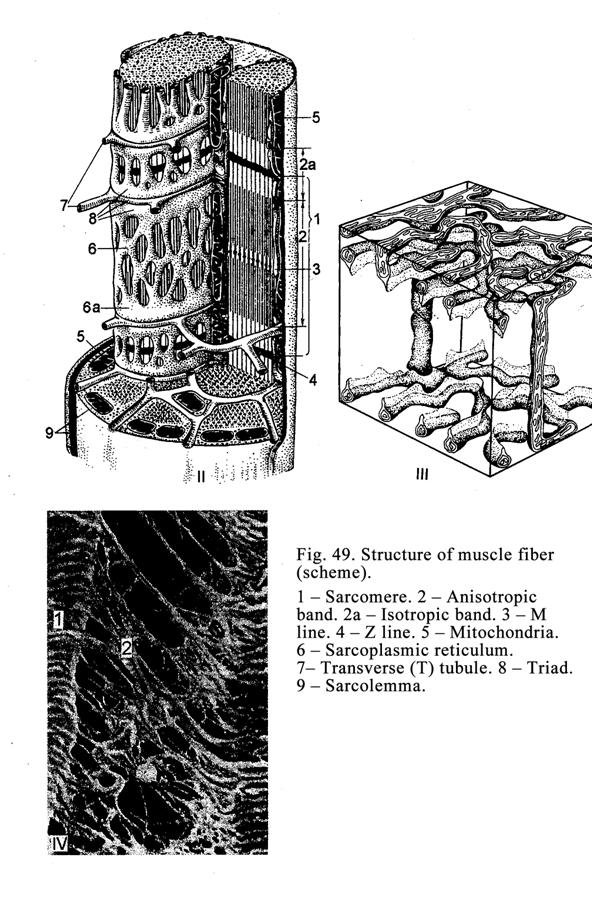
Myofibril Contraction Mechanism
After removal of
tropomyosin molecules from actin filaments myosin molecule heads join with actin
filaments then heads bend dragging actin filaments towards the M-band therefore
the H-band becomes narrower and narrower. At the same time the Z-line
approaches the A-band therefore the I-band also becomes narrower. Thus, during
the contraction of myofibrils both I-band and H-band are narrowed.
After stopping of
nervous impulse moving through the T-canal myofibril relaxes and ions of Ca++
return into terminal cisterns, tropomyosin molecules close actin filaments
again, the I-band and H-band come back in the former position.
Satellite Cells
They are located between
the basal membrane and the plasmolemma of the sarcolemma. Oval cells include
the oval nucleus, surrounded by a thin cytoplasm layer, stained weakly.
Satellite cells are stem cells taking part in the restoring of muscle fibers
after their injury.
Organization of Muscle Fibers and
Connective Tissue in Muscle
Every muscle is a
special organ having a certain structure and providing the contractive function.
It consists of myofibers. Every myofiber is surrounded by thin
layer of connective tissue, called
the endomysium, in which blood and lymphatic vessels, and nerves are present.
Some myofibers constitute a bunch, surrounded by a thick connective tissue
layer, called the perimysium. The entire muscle is surrounded by connective
tissue called the epimysium.
Joining of Myofibers with Tendon
Collagen Fibers
Myofiber ends have pits
in which tendon collagen fibers along with reticular fibers extend. Reticular
fibers penetrate into the myofiber sarcolemma basal membrane and attach to the
plasmolemma by molecular junction. Then the reticular fiber returns into the
pit and interlaces with the collagen fiber joining the latter with the
myofiber. Collagen fibers form the tendon that is attached to the bone
skeleton.
Myofiber Types
There are 2 basic types
of myofibers: 1) type I (red muscle fibers) and 2) type II (white muscle
fibers). They have different speed and strength of contraction, different
contents of myoglobin and glycogen, and different activity of enzymes.
Fiber type I contains much a myoglobin
(therefore the fiber is red) it has a high activity of the enzymes of
mitochondria, possesses the ability to contract without fatigue, its ATP enzyme
is slowly, their contraction is weak.
Fiber type II contains less myoglobin (therefore
this fiber is white) it has a low activity of the enzymes of mitochondria, it
does not posses the ability to contract without fatigue, its ATP enzyme is
rapid, its contraction is fast but it rapidly experiences fatigue.
Red muscle fibers are
innervated by the slow type of neurons while white muscle fibers are innervated
by the rapid type of neurons.
Apart from red and white
muscle fibers there exist intermediate types possessing the properties of both
type I and type II. Every muscle includes all types of fibers. The amount of
these types may be various depending on physical work.
Skeletal Muscle Regeneration
After the rapture of
muscle fibers their ends undergo necrosis. At the same time macrophages move to
the ends to engulf the necrotic parts to clean them. After it the process of
regeneration occurs by two manners: 1) the activity of myofiber organelles
becomes high to form the muscular bud on the rapture ends of myofiber and 2)
satellite cells produce new myofibers.
The first manner is characterized by the hypertrophy
of rough ER synthesizing proteins of myofibrils, components of the
intercellular membranes within myofibers, and sarcolemma. It leads to thickening
of the fiber ends to form muscular buds. The latter grow and grow to join with
each other as a result the muscle fibers are replaced. But at the time of the
myofiber replacement the connective tissue is formed by fibroblasts between the
myofiber ends as a result the connective tissue scar is formed in the place
where the muscular buds are joined.
The second manner is
characterized by the participation of satellite cells in the process of
myofiber regeneration. When the muscle fiber is ruptured, satellite cells leave
the injured myofibers and undergo differentiation as a result they convert in
myoblasts, which fuse with each other to form myotubes. The latter convert in
new myofibers. Some myoblasts join with muscle buds. Thus, at the time of
regeneration the existing myofibers are restored and new myofibers are formed.
The skeletal muscle is
supplied by motor and sensitive nerve fibers ending by nervous ends.
Motor Nerve Ends (Neuromuscular
Terminals)
The terminals are ends
of motor neuron axons. The neurons are located in the spinal cord ventral
horns. The ends of axons, going to the muscle fiber, are divided into some
branches. The branches perforate the sarcolemma basal membrane and immerse
along with plasmolemma into the sarcoplasm as a result the neuromuscular
terminal is formed. The neuromuscular terminal includes 2 parts (poles): 1) the
nervous pole and 2) the muscular pole. Between the poles there is the synaptic
gap. The nervous pole (presynaptic region or motor neuron axon ends) contains
mitochondria and presynaptic vesicles filled with neurotransmitter called the
acetylcholine. The muscular pole contains mitochondria and nuclei but
presynaptic vesicles and myofibrils are absent. The synaptic gap is about 50
nm. It is limited by the presynaptic membrane (plasmolemma of axon) and
postsynaptic membrane (plasmolemma of the myofiber sarcolemma). The
postsynaptic membrane forms the folds making the secondary synaptic gaps. On
the external surface of the postsynaptic membrane there are receptors for acetylcholine.
Neuromuscular Terminal Function
When the nerve impulse
travels along the axon plasmolemma (presynaptic membrane), acetylcholine is
released from presynaptic vesicles into the synaptic gap and is received by the
postsynaptic membrane receptors. It leads to high permeability of the membrane
(the myofiber sarcolemma plasmolemma) as a result Na+ ions pass from
the external surface of the plasmolemma on its internal one but K+
ions pass from its internal on the external surface to form a wave of depolarization
or nervous impulse. After arising of the impulse acetylcholine esterase,
located in the postsynaptic membrane, destroys acetylcholine as a result the
impulse passage through the synaptic gap stops.
Neuromuscular Spindles
Sensitive nerve fibers
(dendrites of sensitive neurons of spinal ganglions) end on myofibers by
sensitive nervous terminals called the neuromuscular spindle. The latter is
covered by a connective tissue capsule. Within the capsule there are 2 type
intrafusal fibers: 1) intrafusal myofibers with a nuclear bag (nucleus bag
fiber, its central part is thickened where some nuclei are present) and 2)
intrafusal myofibers with a nuclear chain (in central part of the fiber the
nuclei are arranged in chain). Nucleus bag fibers are longer and thicker, than
nuclear chain fibers.
Thick nerve fibers enter
the neuromuscular spindle. The fibres surround both intrafusal myofibers (with
nuclear bag and nuclear chain) to form annulospiral nerve terminals. Thin
nervous fibers, entering the neuromuscular spindle, end on myofibers with the
nuclear chain to form branches of the nerve terminal.
Neuromuscular terminals
are present in neuromuscular spindles. They are terminated on the ends of
intrafusal myofibers where myofibrils are also present. The contraction of
intrafusal myofibers is weak and not summarized with the contraction of the
extrafusal myofibers.
Neuromuscular spindles
provide the control of speed and force of the muscle strain. If the myofiber
strain force threatens to break, the myofiber receives depressing impulses from
the nervous system.
Cardiac Muscle
This is derived from
visceral mesoderm. From the latter 2 plates are formed: right and left. The
cells of the plates are divided into 2 groups. From the first group mesothelium
of the epicardium is formed, from other group contractile cardiac myocytes,
pacemaker cells, transitional myocytes,
Purkinje fiber cells, and natriuretic hormone produced cells are
derived.
The Structure of Cardiac Myocytes
Cardiac myocytes are cylindrical
their length is 50-120 mm their
diameter is 10-20 mm. The
cardiac myocyte ends are joined with each other to form a functional
(contractile) cardiac myofiber. Cardiac myocyte joining places are called
intercalated discs. In discs there are invaginations, loci of the actin
myofilament attachments, desmosomes, and nexuses. Nexuses (Communicating
junctions) provide the exchange of some substances between cardiac myocytes.
Cardiac myocytes are covered by the sarcolemma consisting of the external (basal)
membrane and the plasmolemma. Between the cardiac myocyte sides of adjacent
myofibers there are anastomoses.
One or two nuclei of
cardiac myocytes are oval, usually characterized by polyploidy, located in the
central cell part. Some cell organelles develop weakly (rough ER, the Golgi
complex, and lysosomes); other organelles develop well (mitochondria, smooth
ER, and myofibrils). In the cell oxyphil cytoplasm there are glycogen,
myoglobin, and lipids. The structure of myofibrils is similar to that of the
skeletal muscle. Actin filaments form the I-band, divided by the Z-line, myosin
filaments along with the ends of actin filaments form the A-band, divided by
the M-line. In the A-band the middle part the H-band, limited by actin filament
ends, is seen.
The cardiac muscle
myofibers differ from those of the skeletal muscle in that the former consists
of many cells (cardiac myocytes), have anastomoses, their nuclei are located in
the cell central part (in skeletal myofibers the nuclei are found in their peripheral
part), the T-tubule diameter is larger because its wall includes both
plasmolemma and the basal membrane of the sarcolemma (T-tubule of the skeletal
myofiber only includes the plasmolemma).
The contracting
mechanism of cardiac myofibrils is similar to that of skeletal myofibrils. Conductive
cardiac myofibers are characterized by large diameter (50 mm), light cytoplasm, central or
peripheral sitting nuclei, less quantity of myofibrils, weakly developed
intercalated disces. Their intercalated disces contain desmosomes,
invaginations, nexuses, and actin myofilament attachment places. The T-tubules
are absent in conductive cardiac myocytes. The latter have the ability to join
not only by their ends, but also by their lateral surfaces.
Conductive myofibers
provide transport and transmit impulses to contractive cardiac myofibers.
Endocrine
Cardiac Myocytes
They are located in
heart atriums only, have more branches, than contractive cardiac myocytes.
Myofibrils, T-tubules, and intercalated discs are developed weakly in these
cells but rough ER, the Golgi complex and mitochondria are developed well.
Their cell cytoplasm contains secretory granules. These cells release the
natriuretic hormone. This regulates cardiac muscle contraction, the circulation
fluid volume, the blood pressure, and diuresis.
Cardiac muscle
regeneration may
only be physiological intracellular. If cardiac muscle myofiber is destroyed,
it is not restored but is replaced by connective tissue.
CHAPTER 8
THE NERVOUS TISSUE
The nervous tissue
consists of neurons and neuroglia. Neurons posses 4 properties: 1) they can
perceive stimulation, 2) they can stimulate themselves, 3) they can elaborate
nervous impulses, and 4) they have the ability to transmit the impulse to other
neurons or the functional organ. The neuroglia creates the condition for the
development and function of neurons. The neuroglia provides the following
functions: 1) the trophic function, 2) the insulating function, 3) the
protective function, 4) the secretion function, 5) the barrier function, 6) the
supporting function, 7) the exchanging substance function, 8) they take part in
the water and salts exchange, and 9) the growth factor releasing function.
The Nerve Tissue Origin
The nerve tissue is
derived from the nervous crest, nervous plates, and nervous tube. The nervous
crest is formed when the edges of nervous grooves join to form nervous tube.
The group of cells not included in the nervous tube and resting ectoderm
constitutes the nervous crest sitting between the nervous tube and the
ectoderm. Spinal ganglions, autonomic nervous system ganglions, and some head
ganglions are derived from the nervous crest.
The nervous plates are
thickening of the ectoderm near the nervous tube anterior end. They take part
in V, VII, IX, and X pairs of cranial ganglion formations.
The brain, spinal cord,
and eye retina are derived from the nervous tube. The nervous tube cells are
divided into two groups. The first group of cells is called neuroblast from
which neurons are formed; the second group of cells is called glioblasts, from
which glial cells are formed.
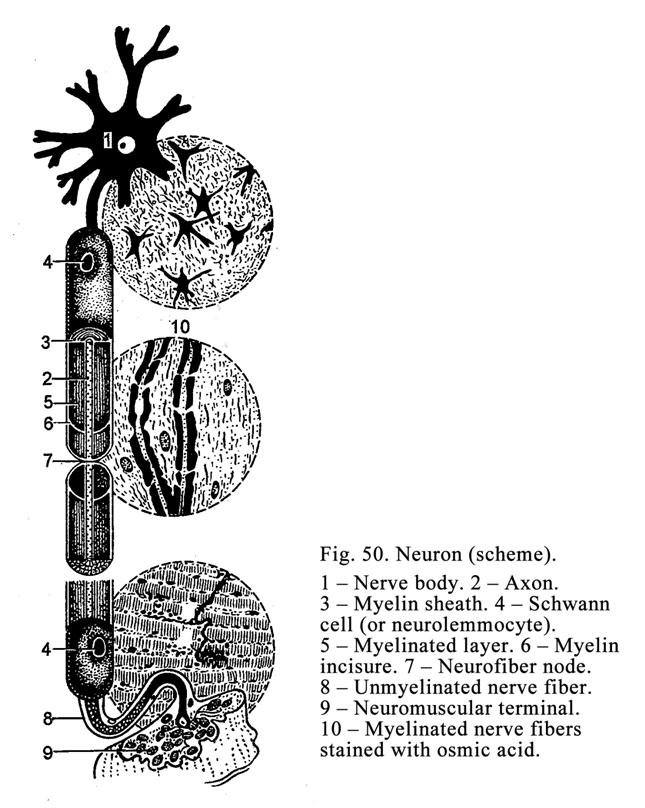
The Neuron Structure
Neurons (Fig. 50) are
4-140 mm in diameter. They have variable
shape (pyramidal, stellate, spindle-like, round, etc.). Every neuron consists
of the cell body, which gives off processes of variable length (from same mm to
Classification of Neurons According
to Their Structure (According to the Number of Processes)
According to the number
of neuron processes neurons (Fig. 51) are divided into 1) unipolar (have 1
process, it may by in embryonic life), 2) bipolar (have 1 axon, and 1 dendrite,
it is seen in the eye retina and spiral ganglion), 3) multipolar (have 1 axon
and some dendrites present in the brain and spinal cord), and pseudounipolar
(in fact they are bipolar neurons because their process are divided into the
first process, serving as an axon and second process serving as a dendrite).
Classification of Neurons According
to Their Function
They are classified into
1) sensitive neurons, their dendrites end by receptors (sensitive nerve ends),
2) efferent (motor) neurons, their axons terminate by motor ends or
neurosecretory ends (terminals) on organs, and 3) intercalated (associative)
neurons join two neurons with one another to transmit impulses from the first
neuron to the second one.
Neuron Nuclei
Round light nuclei are located in
the cell center or the periphery of neurons. The nuclei contain euchromatin and
prominent nucleoli. Every neuron has usually one nucleus except neurons of some
autonomic ganglions situated near the uterine cervix and prostate. The
neuron cell membrane provides the barrier, exchange, receptive, and impulse
conducting functions. Nervous impulses appear when the cell membrane undergoes
the action the neurotransmitters activating its permeability as a result Na+
ions pass from the external surface on the internal cell membrane surface, but
K+ ions pass from the internal surface on the external surface to
provide the nervous impulse (or depolarization wave), which travels rapidly
along the cell membrane of the cell body or its processes.
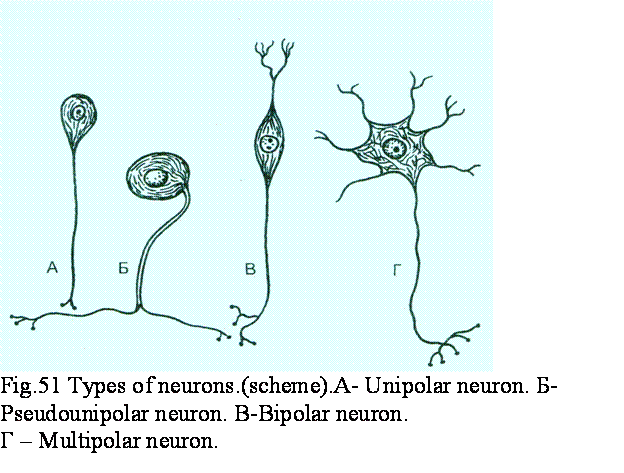
Neuron Cytoplasm
The neuron cytoplasm
contains many mitochondria, rough ER, the Golgi complex, lysosomes, and special
organelles called neurofibrils, forming the cytoskeleton (Fig. 52). A number of
mitochondria are located in the cell body (perikaryon) and their processes are
particularly in their terminals. The Golgi complex surrounds the nucleus and
has the regular ultramicroscopic structure. Rough ER is very well developed. It
forms clumps in the cell body and its dendrites. The clumps, stained by basic
dyes (toluidin blue, tionin, and ctr.), are called the basophilic substance or
Nissl substance. The Nissl substance (or chromatophilic) is absent in axons.
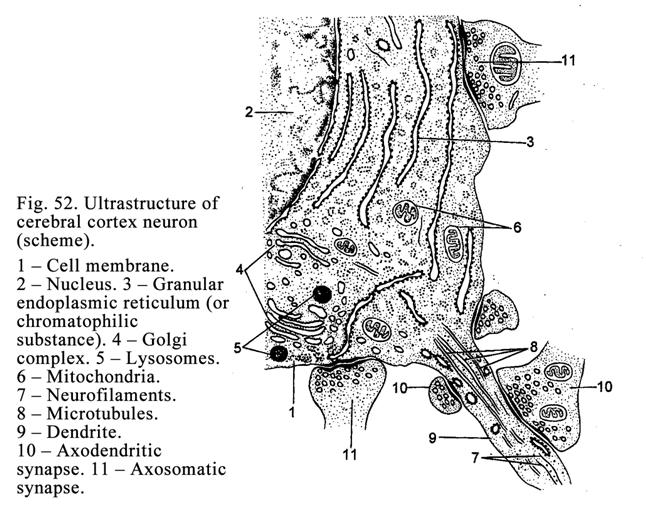
When neuron action is
active, the chromatophilic substance disappears from the neuron. This process
is called chromatolysis.
Neurofibrils
Silver stains
neurofibrils in dark-brown colour. In the neuron body neurofibrils have
different directions but in its processes they have parallel direction.
Neurofibrils are made up of nerve filaments (are 6-10 nm in diameter) and nerve
tubules (are 20-30 nm in diameter) forming the cytoskeleton. They take part in
the intracellular movement of various substances.
Nerve Cytoplasm Flow
It is neuron cytoplasm
movement through cell processes from or to the cell body. There are four neuron
cytoplasm flows: 1) slow axoplasmic flow from the body towards the axon end is
characterized by transport of mitochondria, vesicles, membrane structures, and
enzymes activating the transmitter synthesis (its velocity is 1-
Neuroglial Cells
They are classified into macroglial
and microglial ells (Fig. 53). The latter are represented by glial
macrophages or small glial cells developed from blood monocytes and
performing the phagocytize function.
Macrophages are branching cells. Their cell body gives off some short
processes, which further undergo ramify. The macroglial cells are
subdivided into 3 types: 1) ependymocytes, 2) astrocytes, and 3)
oligodendrocytes. Ependymocytes (ependymal cells), lining the brain ventricle
lumen and the spinal cord central canal, are similar to superficial epithelial
cells and divided into 1) choroid (cuboidal) ependymocytes and 2) columnar
ependymocytes. Both the former and latter have the apical and basal surfaces
(parts). On the apical (luminal) surface of the ependymocytes during embryonic life
there are cilia, which disappear after birth but they are seen in the third
ventricle aqueduct after birth. The columnar ependymocyte basal part gives
off the process, penetrating the brain thickness (or spinal cord thickness) and
takes part in superficial limiting glial membrane formation. Thus ependymocytes
provide the supporting, limiting, and barrier functions. The group of
ependymocytes takes part in commissural organ formation. They provide the
secretory function.
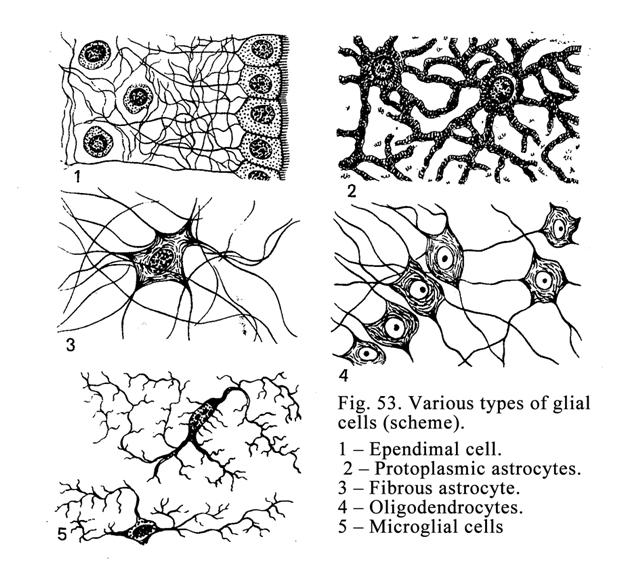
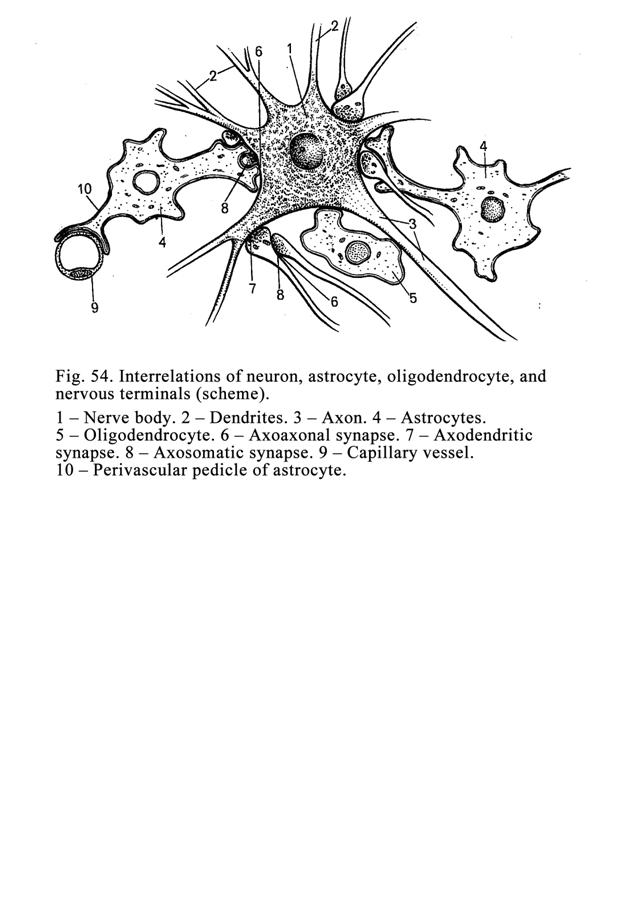
Cuboidal ependymocytes
are located in the brain ventricle wall where the vascular plexuses are
present. These cells basal part has striation (cell membrane basal part folds).
It testifies that these cells provide the cerebral fluid release.
Astrocytes are divided into 1) protoplasmic
astrocytes (Fig. 54, 4) and 2) fibrous astrocytes. Protoplasmic astrocytes are
predominantly present in the grey matter of the brain or the spinal cord. They
give off some thick short processes that are divided into the secondary
processes. The fibrous astrocytes are predominantly located in the white matter
of the brain or spinal cord. From their round cell body a number of long
non-branched processes arises penetrating the white matter and taking part in
the superficial limiting glial membrane formation. Some cell processes reach
blood vessels to form perivascular limiting glial membrane (Fg. 54, 10). Thus,
astrocytes take part in the blood-brain barrier formation.
Astrocytes are
responsible for: 1) supporting of nerve cells, 2) the formation of blood-brain
barrier, 3) participation in the neurotransmitter exchanges, and 4)
participation in the exchange of water and salts, 5) the growth nerve factor
release.
Oligodendrocytes, located in the brain and spinal
cord, surround the neuron bodies and accompany neuron processes (Fig.55). The
Schwann cells (special oligodendrocytes), present in nerves, nervous ganglions,
and nerve ends, are derived from the nervous crest. Depending on the situation,
the oligodendrocytes have various shape, structure, and function. For example,
in the brain they are round or angular.
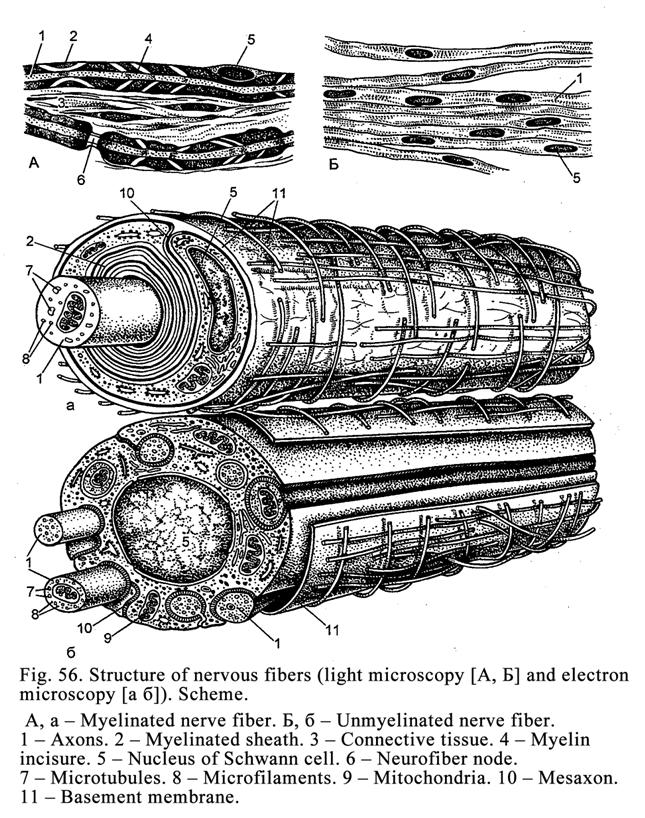
Their bodies give off
some short processes. When the neuron process is in the brain or spinal cord,
the oligodendrocyte shape is flattened. At this cause they are called the
Schwann cells. These also form sheathes round neuron processes in the
peripheral nerves. Here the Schwann cells provide the trophic and insulation
functions and also takes part in the regeneration of nerve fibers after their
injury. In peripheral nervous ganglions oligodendrocytes assume round or oval
shape and surround the neuron body. At the same cause the Schwann cells are
called the satellite cells. In
receptors (tactile corpuscle, lamellated corpuscles) are oval shaped and called
sensitive cells.
Nerve Fibers
Nerve fibers (Fig. 56}
are processes of neurons (dendrites or axons) surrounded by sheath consisting of
the Schwann cells. The axon or dendrite of the nerve fiber is called the axis
cylinder. Depending on the nerve fiber sheath structure of fibers are
divided into myelinated and nonmyelinated nerve fibers. The myelinated fiber
sheath includes the myelin layer. It is called the myelinated nerve fiber. If
the myelin layer in nerve fiber sheath is absent, that is called the
nonmyelinated nerve fiber.
Nonmyelinated Nerve Fibers
They are located in the
peripheral autonomic nervous system. The nonmyelinated nerve fiber sheath is
the cord of the Schwann cells, in which the cylinder axis invaginates. The
nonmyelinated nerve fiber can include some axis cylinders (similar to a cable).
The axis cylinder can enter from one to another nerve fiber.
Nonmyelinated Nerve Fiber Formation
During the nonmyelinated
fiber formation the neuron process (axon or dendrite) invaginate the cord
consisting of the Schwann cells (along with the cell membrane of the Schwann
cells). That is why the process (axon or dendrite) comes to be suspended by the
fold of the Schwann cell membrane. The fold is called the mesaxon. The entire
nonmyelinated nerve fiber is covered with the basement membrane.
Myelinated Nerve Fibers
They are predominantly
localized in the somatic nervous system. They are thicker than nonmyelinated
nerve fibers (about 20 mm in
diameter). Their axis cylinder is also thicker. The myelinated fiber stained by
osmium assumes a black-grey colour. After staining 2 layers are seen in nerve
fiber sheath: the internal grey layer, consisting of myelin, and the external
layer, consisting of the cell membrane, the cytoplasm, and nuclei of the
Schwann cells, called the Schwann sheath, covered by the basement membrane. In
the internal myelinated grey layer the oblique incisures, called the myelinated
incisures, are seen. Along the nerve fiber there are constrictions through that
the myelin layer does not pass. The constrictions are called the neurofiber
nodes (the nodes of Ranvier). Through the nodes only Schwann sheath
(neurilemma) and the basement membrane surrounding the nerve fiber pass. The
neurofiber node is the boundary between the adjoining Schwann cells. Here short
and thin (about 50 nm in diameter) projections (finger-like processes) extend
between the same processes of the adjoining Schwann cell.
The nerve fiber part
between two nodes is called the internodal segment. The segment consists of one
Schwann cell.
The internal grey
(myelinated) substance of the nerve fiber sheath is mesaxon located around on
the axis cylinder.
Myelinated Nerve Fiber Formation
First the myelinated
nerve fiber formation is similar to that of the nonmyelinated fiber. The neuron
process invaginates into the Schwann cell cord. This process becomes suspended
by the fold of the Schwann cell membrane (mesaxon).
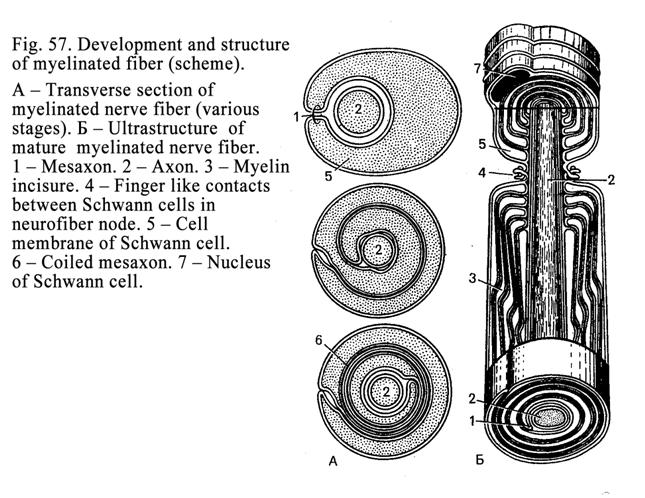
The mesaxon becomes
greatly elongated to be spirally wound on the axis cylinder (Fig. 57), which is
thus surrounded by several layers of the cell membrane (mesaxon). At the same
time the cytoplasm and nuclei of the Schwann cells are pushed to the periphery
to form the neurilemma. Thus, the myelinated layer of the myelinated nerve
fiber is the mesaxon, wounded on the axis cylinder. The neurilemma is the
cytoplasm, which along with the nucleus is pushed to the Schwann cell
periphery.
Distinctions
between Myelinated and Nonmyelinated Nerve Fibers
The myelinated and
nonmyelinated nerve fibers have distinctions in the structure and function. For
example, the myelinated nerve fibres impulse velocity is 5-
With the help of the
electron microscope (EM) the distinction between the myelinated and
nonmyelinated nerve fibers is seen well. The axis cylinder of the myelinated
fiber is surrounded by a number of mesaxon layers while in the nonmyelinated
nerve fiber they are absent.
Regeneration of Neurons
If a neuron is injured,
it is not regenerated, if its process, included in peripheral nerve, is cut,
the latter is regenerated (Fig. 58). After the peripheral nerve (or nerve
fiber) is cutting it has two parts: proximal and distal. The latter is
separated from the cell body by cutting.
In the distal part of the nerve
fiber two processes occur: 1) anterograde degeneration and 2) regeneration.
First anterograde degeneration begins. At the same time the Schwann cells
become swollen, the myelinated layer is dissolved, axis cylinder is a
fragmented, ovoid droplet, consisting of myelin and cylinder axis fragment, are
formed. In 2 weeks ovoid droplets are dissolved the Schwann cells are persists.
The Schwann sells continue dividing to form cords (bands). After this process
the axis cylinder proximal part end becomes swollen to form the growth bud. The
latter slides along the Schwann cell cord (band). At the same time between the
proximal and distal parts of the injured nerve the glial scar is formed
preventing to the slide of the growth bud. Therefore some nerve fiber growth
buds cannot pass through the scar. Thus, after cutting the peripheral nerve the
organ nerve supply is replaced incompletely.
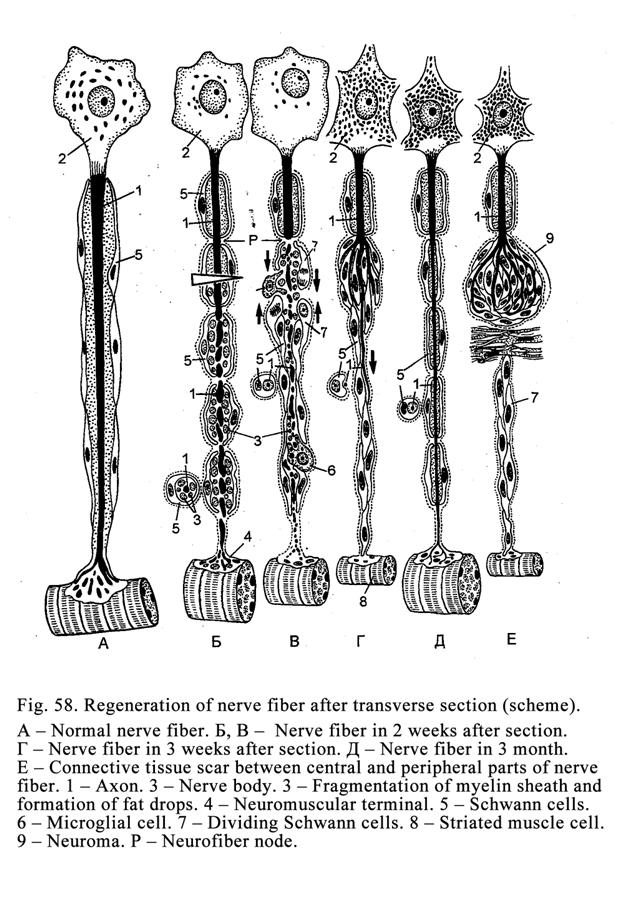
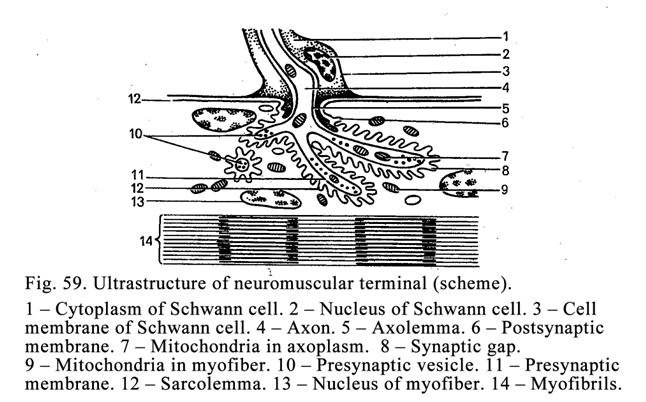
The axis cylinder,
passing through the scar, invaginating the Schwann cell cord to form mesaxon.
The mesaxon is elongated and wound around the axis cylinder to form the myelin
layer. At the place, where the nerve ending must be formed, the axis cylinder
growth delays. After it the nerve fiber terminal and other components of nerve
endings are formed.
Nerve Endings
Nerve endings are classified
into effector (motor) nerve endings and receptors (sensitive nerve endings),
and neuron synapses.
Effector Nerve Endings
They are divided into
motor or neuromuscular terminals and neurosecretory terminals.
Neuromuscular Terminal (Fig. 59)
They are formed as
follow. When the nerve fiber approaches the myofiber the former loses the
myelin layer of its sheath. The resting Schwann sheath fuses with the
sarcolemma basal membrane. The axon (axis cylinder) is divided into some
terminals. The axis cylinder terminals, losing the Schwann sheath, along with
plasmolemma of sarcolemma invaginates the myofiber sarcoplasm. Thus, every axon
terminal is covered with the plasmolemma due to it two poles are formed: neural
and muscular.
Neural Pole
It is represented by the
terminals of the axon, which are covered by the cell membrane (axolemma). The
terminals contain mitochondria and vesicles including the neurotransmitter
called the acetylcholine.
Muscular Pole
This is a part of the
sarcoplasm of the myofiber without myofibrils. Here many mitochondria and
nuclei are present.
Between both nervous and
muscular poles there is the synaptic gap, limited by the presynaptic membrane
(the axon cell membrane) and postsynaptic membrane (plasmolemma of sarcolemma).
The distance between these membranes is about 50 nm. The plasmolemma
(postsynaptic membrane) makes folds called second synaptic gaps. The
postsynaptic membrane has acetylcholine receptors (the receptors can hold a
acetyl choline) and an enzyme destroying acetylcholine (acetylcholine
esterase).
The nerve impulse can pass through
the synaptic gap from the nerve pole to the muscular pole. When the nerve
impulse travels along the axon cell membrane (presynaptic membrane), the vesicles,
containing acetylcholine, move to presynaptic membrane, fuse with it,
and release acetylcholine into the synaptic gap. The released acetylcholine
attaches to receptors so the plasmolemma (postsynaptic membrane) permeability
becomes high. As a result
Na+ ions pass from plasmolemma external surface to its
internal surface wile K+ ions pass from the internal surface to the
external surface so the wave of polarization (nerve impulse) occurs. The
impulse travels along the myofiber plasmolemma and T-tubules, as a result the
myofiber contracts. Thereafter acetylcholine esterase, present in the
plasmolemma, destroys acetylcholine of the receptors.
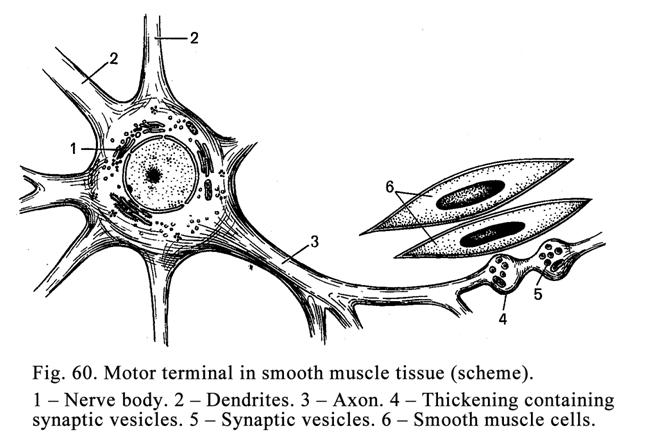
The smooth muscle cells motor nerve endings (Fig. 60) are terminals of neuron axons
located in autonomic ganglions. In the terminal there are swelling containing
neurotransmitters. When the nerve impulse reaches the terminals,
neurotransmitters are released from the swellings and diffuse to the smooth
muscle cells through the connective tissue.
Neurosecretory Terminals
These are efferent neuron
axons, located in autonomic nerve ganglions, having the structure similar to
that of the smooth muscle terminals but ending on gland cells. When a nerve
impulse reaches the nerve end, the neurotransmitter released from the terminal
swelling is caught by gland cell receptors. It leads to the activation of
adenylate cyclase. The latter influences the synthesis of cyclic adenosine
monophosphate (cyclic AMP). Cyclic AMP stimulates cell enzyme activities and
the cell function is altered.
Receptors (Sensory Terminals)
They are classified into
exteroceptors (localized on the skin or mucous membranes) and interoceptors
(localized on the viscera). Interoceptors include proprioceptors, ending on
skeletal muscles, joints, tendons, and ligaments.
From the functional
point of view receptors can be classified into pressure receptors,
thermoreceptors, mechanoreceptors, and chemoreceptors. Sensitive neuron
dendrites of peripheral sensitive ganglions end by receptors.
Receptors may be
classified on the basis of their structure (Fig 61). There are free nerve
endings and non-free nerve endings. Free nerve endings are predominantly
located in the epithelium. Their terminals, devoid of the sheath, are ramified
between epithelial cells. For example, the skin epidermis contains special
sensory epithelial cells called the Merkel cells. The nerve fiber terminal,
reaching the Merkel cell, comes in contact with it. Between the former and
latter the Merkel disc is located. The free nerve endings can be stimulated by
temperature, touch, and pain. Non-free nerve terminals are further divided into
encapsulated and non-encapsulated corpuscles.
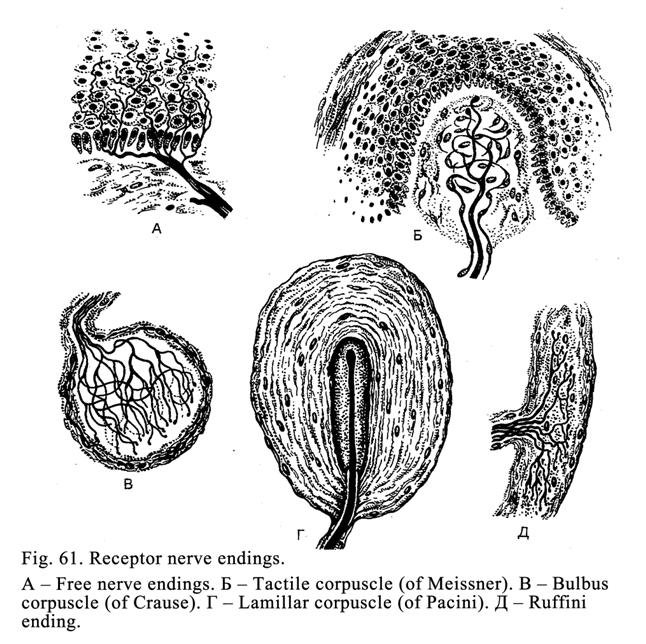
Non-
encapsulated Nerve Ending
Their axis cylinder,
covered with myelin sheath, ramifies similar to a bush. The endings are usually
located in connective tissue.
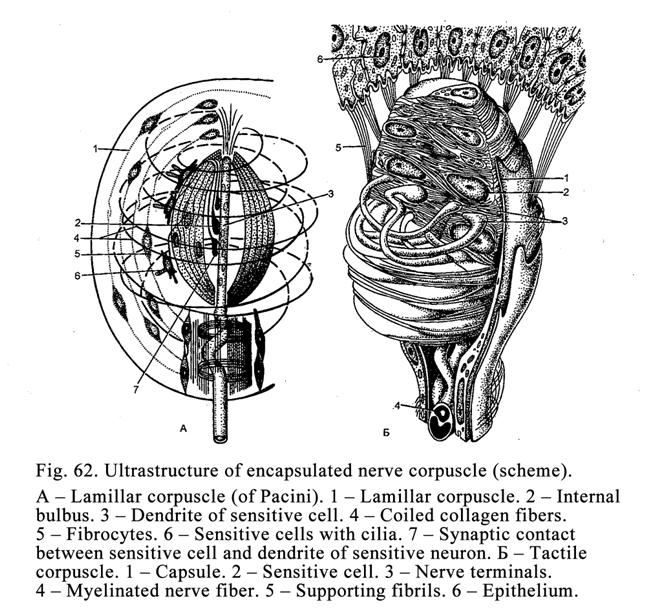
Encapsulated Nerve Corpuscles (Fig.
62)
They are covered with a
connective tissue capsule. This group of corpuscles includes 1) lamellar
corpuscles, 2) tactile corpuscles, 3) neuromuscular spindles, and 4)
neurotendinous spindles.
Lamillar Corpuscles (Fig. 62A)
They are located in deep
layers of the skin and viscera stimulated by pressure. Lamillar corpuscles
consist of collagen fiber layers and fibrocytes (external bulb) and the
internal bulb including the branched axis cylinder coming in contact with
sensitive cells (the Schwann cells).
Tactile Corpuscles (Fig. 62Į)
They are seen in
relation to dermal papilla. They are covered by a thin connective tissue
capsule. The axis cylinder, entering the capsule, ramifies and come in contact
with sensitive cells. In the genitalia region there are genital corpuscles.
Their structure and function are similar to those of the tactile corpuscles.
Neuromuscular Spindles Fig. 63)
The spindles, covered by
connective tissue capsule, are located in the skeletal muscle. They contain
short and thin intrafusal muscle fibers (intrafusal myofibers). There are 2
types of intrafusal myocytes (myofibers): 1) myocytes with the nuclear bag
(nuclear bag fiber) and 2) myocytes with the nuclear chain (nuclear chain
muscle fibers). The former are longer and thicker, the latter are shorter and
thinner. The middle of the intrafusal myocyte with the nuclear bag, containing
some nuclei, is thickened.
In the middle of the
intrafusal myocyte with the nuclear chain the nuclei lie in a single row (there
being no dilation of myocyte).
The intrafusal myocyte
parts, where the nuclear bag or nuclear chain is present, are sensitive parts.
Here myofibrils are absent. The peripheral parts (ends) of the intrafusal
myocytes contain myofibrils and neuromuscular terminals.
Through the
neuromuscular spindle capsule two types of nerve fibers enter: 1) thick nerve
fibers (17 mm in diameter), which wind spirally
around the nuclear region of both types of intrafusal myocytes and are, therefore,
referred to as annulospiral nerve
terminals, and 2) thin nerve fibers (8 mm in diameter), called branches of nerve
terminals, are only seen in the nuclear chain muscle fiber. The annulospiral
nerve terminals are concerned with the length and force of the muscle tension
while branches of nerve terminals are responsible for myofiber length. If the
myofiber tension is too high and rapid, nerve terminals send impulses to the
central nerve system so the muscle contraction stops.
Synapses
They are divided into
electrical and chemical synapses.
Electrical Synapses
Such synapses are
characterized by close a contact between the membranes of the adjoining cells
(Fig. 64 Į). Through
the synapse the impulse can pass in both directions: from the first to the second
cell and vice versa.
Chemical Synapses
Synapses involving the
release of neurotransmitters are referred to as chemical synapses. Through such
synapse the impulse can pass from the presynaptic region to the postsynaptic
one. Chemical synapses (Fig.
Every synapse contains 3
parts: 1) presynaptic region, 2) postsynaptic region, and 3) synaptic gap.
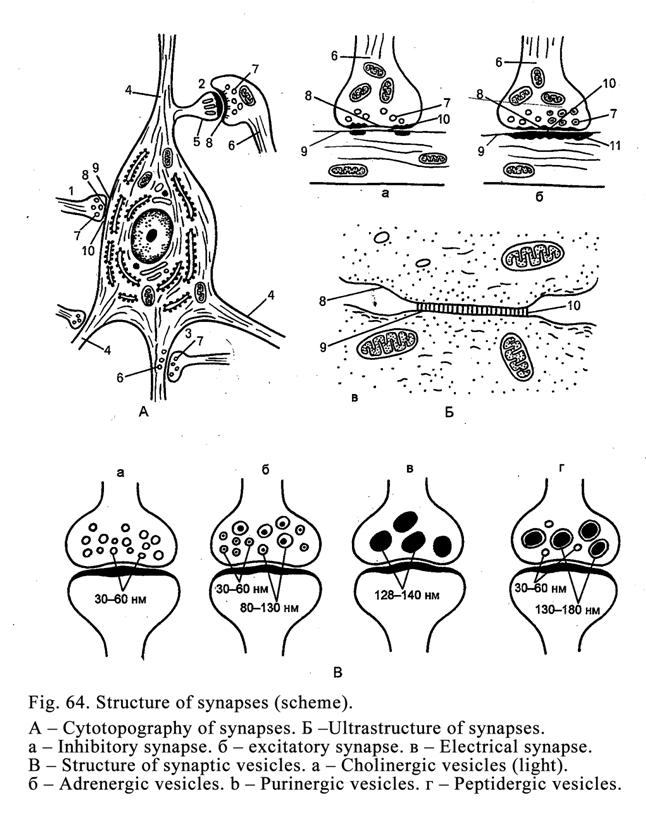
Presynaptic Region
It is always the
terminal of the axon in which there are mitochondria, presynaptic vesicles,
containing neurotransmitters.
Neurotransmitters are
acetyl choline (in cholinergic synapses) and noradrenalin (in adrenergic
synapses). There are others neurotransmitters: dopamine, gama-aminobutyric acid
(GABA), glycine, glutamine, substance P, histamine, serotonin, somatostatin,
and many others. The presynaptic region includes the presynaptic membrane (the
axon cell membrane) containing ion canaliculi.
Postsynaptic Region
This includes the
postsynaptic membrane (the dendrite cell membrane, cell body cell membrane, or
axon cell membrane). The postsynaptic membrane bears acetylcholine receptors
and the enzyme destroying acetylcholine. In the postsynaptic region there is a
dense cytoplasm, a number of mitochondria, but presynaptic vesicles are absent.
Synaptic Gap
The synaptic gap width
is about 20 nm. The gap is limited by both presynaptic and postsynaptic
membrane. The membranes are joined by thin fibrils.
A nerve impulse passes through the
synaptic gap as follows. The impulse, traveling along the axon terminal, moves
Ca++ ions into ionic canaliculi, leading presynaptic vesicles to the
presynaptic membrane. The presynaptic vesicle membranes fuse with the
presynaptic membrane, undergo rapture so the neurotransmitter outflows into the
synaptic gap. The neurotransmitter is caught by postsynaptic membrane
receptors. It leads to a high permeability of the postsynaptic membrane (neuron
cell membrane) as a result Na+ ions pass from the external to
internal surface of the postsynaptic membrane while K+ ions pass to
the apposite direction to provide a low negative electric potential from 70 to
59 mw. It allows nerve impulse formation. After it a special enzyme breaks down
the neurotransmitter and the nerve impulse movement stops.
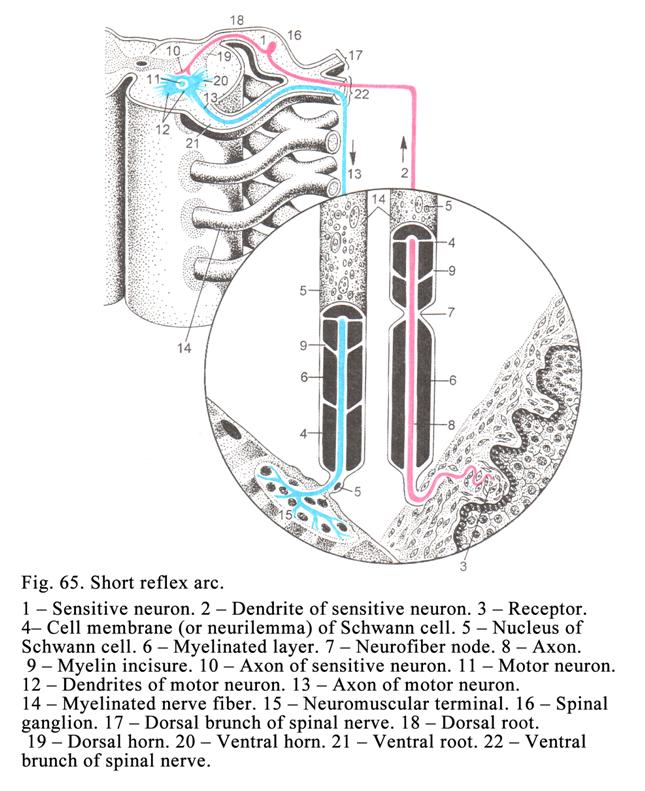
If the synapse is of the
inhibitory type, the neurotransmitter caught by receptor, makes the negative
potential high as a result the nerve impulse cannot pass through the synaptic
gap.
Reflex Arcs
The reflex arc (Fig. 65)
is a system on neurons, joined with each other, to form a chain along which the
nerve impulse moves. The arch always includes one sensitive neuron (it is
always the firs neuron of the reflex arc) and one efferent (motor) neuron (it
is always the last). Simple arc may consist of only two neurons (sensitive and
motor). A complicated reflex arc includes more than two neurons. Other neurons
(apart from the first and the last) are associative neurons (inter neurons).
Impulse Movement through the Reflex Arc
The stimulated receptor
forms a nerve impulse. The impulse travels along the dendrite, body, and axon
of the sensitive (the first) neuron. Then the impulse passes through the
synapse to the second (motor) neuron. The latter transmits the impulse to the
skeletal muscle. This is called the somatic arc. If the impulse from the
autonomic ganglion neuron is transmitted to the smooth muscle or gland, it is
called the autonomic reflex arc.
The Autonomic Nerve System Reflex
Arc
The reflex arc first
neuron may be the same, to that the somatic arc, but associative and efferent
neurons belong to autonomic nerve system. For example, the first neuron sits in
the spinal ganglion, the second neuron localizes in the lateral nucleus of grey
commissure of the spinal cord the third neuron is present in the autonomic
ganglion. The third neuron along with the second neuron constitutes the reflex
arc efferent part.
CHAPTER 9
NERVOUS SYSTEM (SPINAL CORD. PRIPHERAL NERVE & SPINAL GANGLION)
The nerve system is
divided into the central nerve system (CNS) and the peripheral nerve system.
The CNS includes the brain and spinal cord. The peripheral nerve system
includes peripheral nerve ganglions, peripheral nerves, and nerve terminals. On
the basis of functional properties the nervous system is divided into the
somatic and autonomic nerve systems. The somatic nerve system supplies skeletal
muscles. The autonomic nerve system supplies viscera, glands, and the
cardio-vascular system.
Nerve System Origin
The nerve system is
derived from the nervous tube, nervous crest and thickening of the ectoderm
near the brain. From the cranial end of the nervous tube and nervous crest the
brain and cranial nerve ganglions are derived. The spinal cord origin is the
nervous tube caudal part.
The nervous tube cells multiply
resulting in thickening of the tub sides. The thickening consists of three
layers: 1) ependymal layer, 2) mantle layer, and 3) marginal layer. At the same
time the nervous tube has dorsal and ventral plates, anterior, posterior, and
lateral columns (horns). The ependymal layer is the origin of the ependymal
epithelium lining the spinal cord central canal, and brain ventricles. The
mantle layer is the origin of the spinal cord grey matter. The marginal layer
is the origin of the spinal cord white matter. Anterior column cells undergo
differentiation to convert into motor neurons. Their axons form anterior
(ventral) roots. The posterior horn cells differentiate, and convert into
associative neurons. Their axons enter the white matter and run to the brain.
The nervous crest cells travel in the sites where they convert into neurons and
glial cells of the spinal and autonomic ganglions.
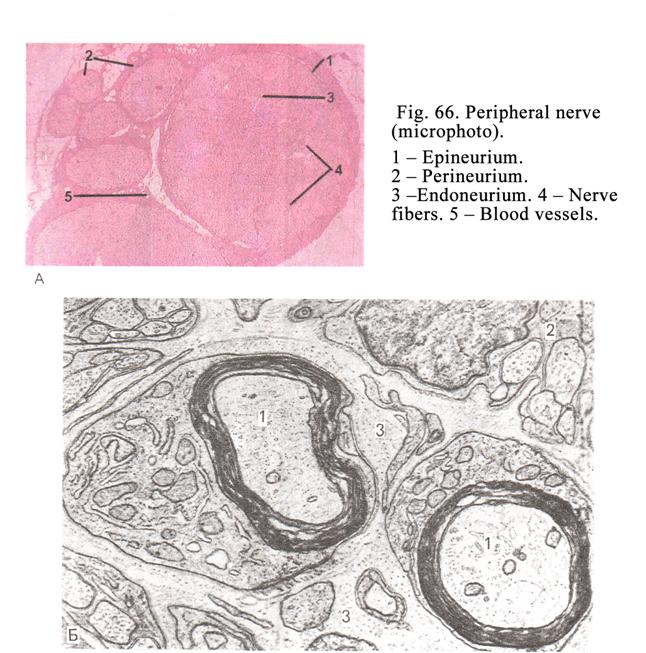
Peripheral Nerves
Peripheral nerves are collections of
myelinated and nonmyelinated nerve fibers (Fig. 66). Some neurons and nerve
ganglions may be present in nerves. In peripheral nerves there are thin layers
of connective tissue. The connective tissue, surrounding the nerve fiber, is
called the endoneurium. The connective tissue, surrounding the nerve fiber
fascicles, is called the perineurium. The connective tissue, surrounding the
entire nerve, is called epineurium. The perineurium is made up of 5-6 layers of
collagen fibers. Between the layers slit-like spaces, lined by the nerve
epithelium, are seen. Within the spaces a fluid circulates. In the perineurium
and epineurium there are blood vessels and nerves.
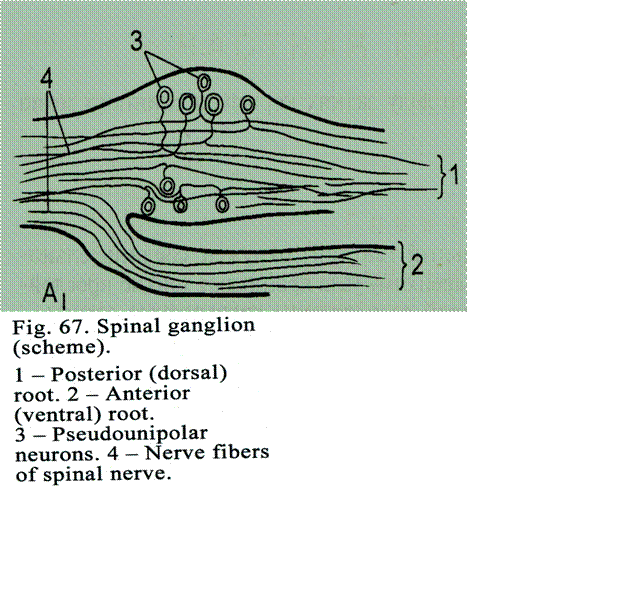
Sensory Ganglia
There are cranial and
spinal sensory ganglia.
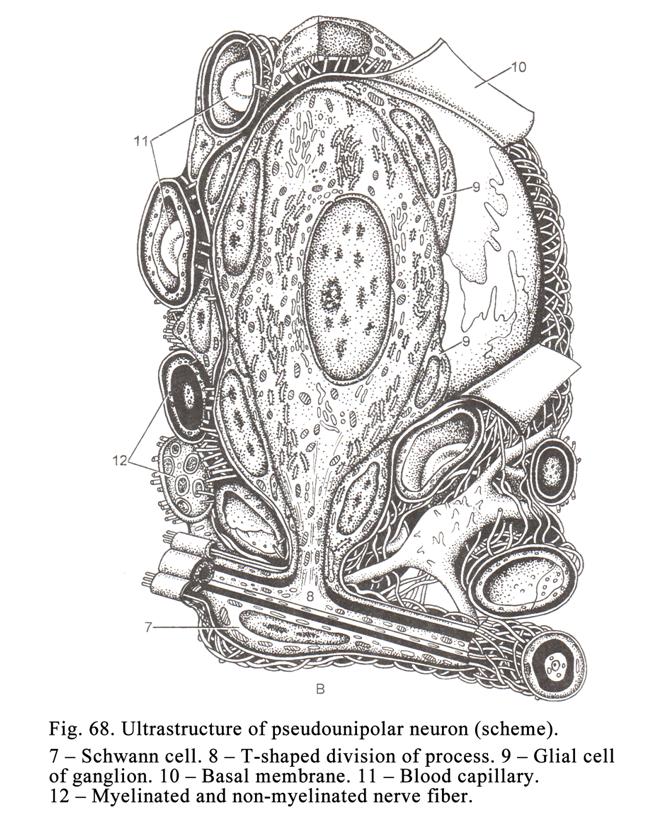
Spinal Sensory Ganglia
The ganglia are located
on dorsal nerve roots. They are closely connected with the spinal nerve and
posterior and anterior roots. The ganglion is covered by the connective tissue
capsule. The ganglion stroma is formed by connective tissue. In the spinal
ganglion the pseudounipolar neurons are present. The round neuron cell body
(Fig 67, 3, Fig. 68)) gives off one process, which some times surrounds the
cell body then divides into two processes. One process is the axon the other
process serves as the dendrite. The neuron cell bodies are predominantly
located on the ganglion periphery; their processes are located in its center.
Every neuron cell body is surrounded by satellite cells and thin connective
tissue capsule. (Satellite cells go into the Schwann cells, covering the neuron
processes; the connective tissue of each neuron is continuous with
endoneurium).
The dendrites of pseudounipolar neurons are
included in the spinal nerve and run to the periphery of the body (the human
body). The spinal nerve consists of the pseudounipolar cell dendrites (sensory
nerve fibers), which join with the motor nerve fibers of the anterior root.
Thus, the spinal nerve is a composition of the sensory and motor nerve fibers.
The pseudounipolar
neuron axons (root nerve fibers), included into the posterior (dorsal) root,
enter the spinal cord. Part of the axons (root nerve fibers) enters the spinal
cord grey matter and comes in contact with its neurons. Some root nerve fibers
(axons), containing substance P and glutamine acid (i.e. neurotransmitters),
are thin (thin root nerve fibers). Thin
root nerve fibers conduct impulses from the skin, and viscera. Other thicker
nerve fibers conduct impulses from the tendons, skeletal muscles, and joins
(proprioceptive nerve fibers).
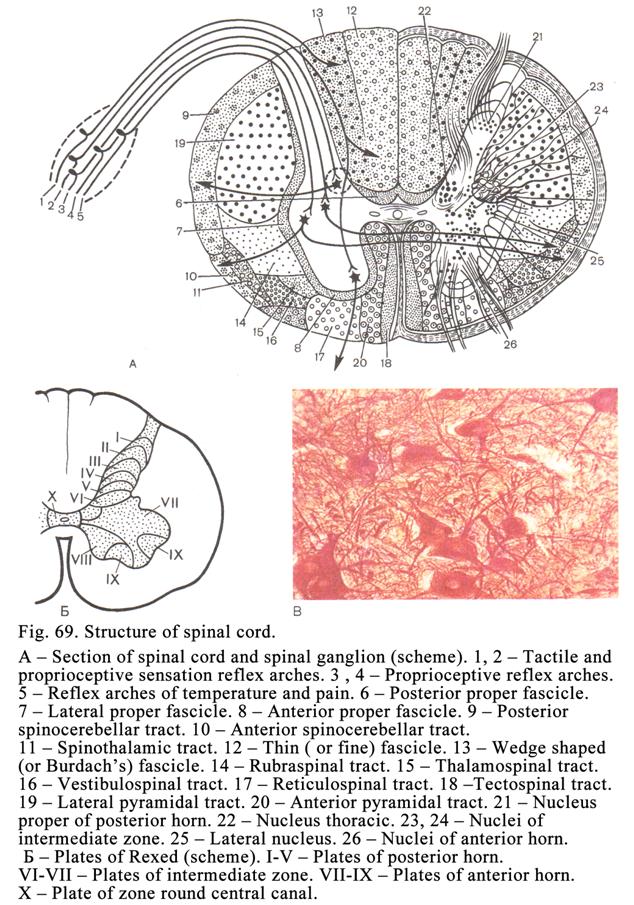
The second part of the
pseudounipolar neuron axons enters the spinal cord white matter to form two
fascicles: thin fascicle and cone fascicle ending on the respective nuclei of
the medulla oblongata.
Spinal Cord
It is located in the spinal
column canal. In the transverse section it is seen that the spinal cord
consists of two symmetric halves (right and left). The boundary between the
halves passes though the dorsal median septum, the spinal cord central canal,
and the ventral medial fissure (Fig. 69).
In the transverse
section it is also seen that the spinal cord consists of grey and white
substance.
Grey Substance (Matter)
It is localized in the
spinal cord central part and looks like a butterfly or letter H. In the grey
substance there are posterior (dorsal), anterior (ventral), and lateral horns
(columns). Between the posterior and anterior horns there is grey commissure.
In the grey substance center the central canal is seen.
From the histological point
of view the grey substance consists of glial cells and neurons along with their
processes covered by a sheath. All neurons of the grey substance are
multipolar. Among neurons are seen some variations of dendrite ramification.
There are spherical, hemispherical, disc-like, conical, and flat neurons.
The grey substance is
conventionally divided into 10 Rexeds plates (Fig.69Į). The dorsal horns include I-V plates. The
grey commissure includes VI-VII plates. The ventral horns include VIII-IX
plates. The tenth plate surrounds the central canal.
Gelatinous Region
It is located in I-IV
Rexeds plates. The gelatinous region neurons produce the encephalin (pain
mediator). In addition, neurons of I-III plates produce 2 mediators
(metencephalin and neurotensin). The
neurons enable inhibition
of pain impulses, conducted by
thin axons of spinal cord neurons, bearing substance P. Neurons of IV plate
produce GABA (inhibit mediator). The neurons, localized in the gelatinous
region, inhibit sensory impulses from the skin and viscera, but scanty impulses
from joints, muscles, and tendons. Neurons, taking part in the conduction of
various impulses, are located in certain spinal cord Rexeds plates.
The skin and visceral
sensitivity are conducted by neurons situated in the gelatinous substance (I-IV
plates). The neurons of the proper nucleus of the posterior horn (IV plate)
conduct pain, temperature, and impulses from the skin and viscera it also
conducts impulses from proprioceptors. Thoracic nucleus neurons (V plate) and
grey commissure medial nucleus neurons (VI-VII plates) provide proprioceptor
conduction.
Spinal Cord Grey Substance Neurons
(Fig. 69A)
The grey substance
contains 3 types of neurons 1) fasciculate neurons (fascicle forming neurons
connecting the spinal cord with the brain), 2) root forming neurons (forming
ventral roots), and 3) small interneurons (neurons connecting spinal cord
segments). The fascicle forming neurons and root forming neurons spinal cord
nuclei are formed. In addition some fasciculate neurons are scattered in the
grey substance. Small interneurons are concentrated in the spongy and
gelatinous substance of the dorsal horns and Cochals nucleus localized in the
ventral horns. They are also scattered in the dorsal horns and grey commissure.
Axons of pseudounipolar neurons of spinal ganglions come in contact with small
interneurons.
Spongy Substance of Dorsal Horns
The substance located in
the dorsal horn distal part consists of interlacing glial cell processes. Here
small interneurons rest. Some scientists call the spongy substance the dorsal
marginal nucleus. They believe that some neuron axons of the spongy substance
reach the thalamus. It is also believed that small interneuron axons come in
contact with the spinal cord own half neurons (associative neurons) or opposite
half (commissural neurons) but their dendrites come in contact with spinal
ganglion pseudounipolar neuron axons (root nerve fibers).
Gelatinous Substance of Dorsal Horns
It is locate near the
spongy substance. Its structure is similar to the latter. The gelatinous
substance includes identical small interneurons providing the same functions.
In the posterior horns and grey commissure associative and commissural small
interneurons are scattered. Associative neurons connect axons of pseudounipolar
neurons with the neurons of their half of the spinal cord, but the commissural
neurons connect them with the opposite half of the spinal cord. A small
interneurons Cochals nucleus connect pseudounipolar neurons with ventral horn
motor neurons.
Spinal Cord Nuclei
Nuclei are aggregations
of neurons having identical structure and function. Almost every nucleus begins
in the cranial part of the spinal cord but its end is in the caudal part of the
spinal cord (similar to the column).
Nuclei Consisting of Fascicle
Forming Neurons
There are 3 nuclei
including the neurons: 1) posterior horn proper nucleus, 2) grey commissure
medial nucleus, and 3) thoracic nucleus. All neurons, included in these nuclei,
are multipolar. These neuron axons run to the brain to form fascicles therefore
they may be refers to as fascicle forming neurons. These neurons are functionally called afferent
associative neurons.
Posterior Horn Proper Nucleus
The nucleus is located
in the posterior horn center. The first part of the axons of nucleus neurons
runs through the anterior grey commissure to the white substance of the spinal
cord opposite half to form the anterior conduction tract traveling to the
cerebellum. The second part of the neuron axons travels to the thalamus. The
nucleus neurons receive impulses from thin axons of spinal ganglion neurons
(thin root nerve fibers), conduct skin and viscera sensitivity, and neurons
receive impulses from thick axons (thick root nerve fibers) conduct
proprioceptive sensitivity (impulses from the skeletal muscle, tendons, and
joints).
Medial Nucleus of Grey Commissure
Ii is located in the
grey commissure near the central canal. Its neuron axons join with the anterior
cerebellum tract. In addition this nucleus includes neurons containing
cholecystokinin, vasoactive intestinal polypeptide (VIP), and somatostatin.
Axons of these neurons run to the lateral nucleus of the grey commissure.
Neurons of the medial nucleus of the grey commissure receive impulses from thin
spinal ganglion neuron axons, sending glutamine acid, substance P. The nucleus
also receives thick axons from the spinal ganglion. Thin axons conduct skin and
visceral sensitivity, thick axons conduct proprioceptive sensitivity.
Thoracic Nucleus
This is located in the
posterior horn medial basal part. The axons its neurons run to the cerebellum
to form the dorsal cerebellum tract of the spinal cord of the same half. The
nucleus neurons receive impulses from thick axons of spinal ganglion neurons
(they conduct proprioceptive sensitivity).
Thus, neuron axons of
all the mentioned nuclei, consisting of fascicle formation neurons, run to the
cerebellum cortex in addition the horn posterior proper nucleus sends their
axons to the thalamus and neurons of medial nucles of grey commiure send axons
to lateral nucleus of grey comissure.
Nucleus Consisting of Root Forming
Neurons
There are 5 nuclei in
the ventral horns and 1 nucleus in the grey commissure (grey commissure lateral
nucleus).
Lateral Nucleus of Grey Commissure
This is located in the
lateral horn. Its neurons belong to efferent associative neurons having a large
size. The nucleus part from the first thoracic segment to the second lumbar
segment belongs to the sympathetic nerve system; the part lower than the second
lumbar segment belongs to the parasympathetic nerve system. The neuron axons
(sympathetic fibers) of the nucleus sympathetic part leave the spinal
cord grey substance along with motor neuron axons to form the anterior
(ventral) roots. Then sympathetic fibers separate from the anterior roots and
run to sympathetic ganglions. The neuron axons of the nucleus parasympathetic
part run to the autonomic (parasympathetic or intramural) ganglion sitting near
different organs (the stomach, spleen, and ctr.). Nucleus neurons have very
active enzymes destroying neurotransmitters.
These neurons are
referred to as root forming neurons because their axons form the ventral roots.
The axons are preganglionic cholinergic myelinated nerve fibers.
The grey commissure lateral
nucleus neurons receive impulses from the medial nucleus of the grey commissure
and from interneurons of the spinal cord. In addition these neurons receive
impulses from thin root nerve fibers, which carry glutamine acid (mediator).
Ventral Horn Nuclei
In the ventral horn
there are 5 nuclei: the lateral anterior nucleus, lateral posterior nucleus,
medial anterior nucleus, lateral posterior nucleus, and central nucleus.
Nucleus neuron axons form the ventral roots. Axons (motor nerve fibers) join
the spinal ganglion neuron dendrites to form
the spinal nerve.
Motor nerve fibers reach the skeletal myofibers to form
the neuromuscular terminals. All 5 nuclei of the ventral horns are motor nuclei
including motor neurons. The latter are the largest neurons of the spinal cord.
They are called root-forming neurons because their axons take part in the
ventral root formation. These neurons belong to the somatic nerve system. Motor
neurons receive nerve impulses from neurons of the spongy substance, gelatinous
substance, the Cochals nuclei, neurons scattered in the grey substance, root
nerve fibers, scattered small internal neurons, and the brain. Owing to it on
the motor neuron body and its dendrites about 1000 synapses are formed.
In the ventral horn there
are lateral and medial nucleus groups. The lateral groups, consisting of motor
neurons, are located in cervical and lumbosacral dilation of the spinal cord.
From the dilation neuron axons run to the upper and low limbs. Medial nuclei
innervate the body muscle.
Thus, the spinal cord
grey substance includes 9 nerve nuclei. Three of them consist of fascicle
forming neurons, connecting the spinal cord with the brain (posterior horn
proper nucleus, thoracic nucleus, and grey commissure medial nucleus), six nuclei,
consisting of root forming neurons, which axons form the ventral roots (5
nuclei of the ventral horn and 1 lateral nucleus of grey commissure).
The small fascicle
forming neurons are scattered in the grey substance. Their axons leave the grey
substance to form their own tracts of the spinal cord. The leaving axons
undergo dividing into ascending and descending branches, which come in contact
with motor neurons at various levels of the spinal cord. Thus, if one small
fascicle forming neuron receives the nerve impulse, it will be transmitted to a
number of motor neurons located in different levels of the spinal cord.
Spinal Cord White Substance
This is represents by
myelinated and nonmyelinated fibers forming the conducting tracts. Every half
of the spinal cord white substance is divided into 3 parts:
1) ventral funicles limited by the
ventral medial fissure and ventral roots, 2) lateral funicles limited by the
ventral and dorsal roots, and 3) dorsal funicles limited by the dorsal roots
and dorsal median septum. The ventral funicles contain descending conductive
tracts connecting the brain with the spinal cord. The dorsal funicles contain
ascending conductive tracts. The lateral funicles contain both ascending and
descending conductive tracts.
There are 5 basic
ascending tracts: 1) fine fascicle, 2) conic fascicle (both fine and conic
fascicle consist of the spinal ganglion neuron axons) ending on the respective
nucleus of the brain stem, 3) ventral cerebellar tract, 4) dorsal cerebellar
tract, and 5) thalamic tract. Three these tracts are located in the lateral
funicle.
The ventral cerebellar
tract and thalamic tract consist of posterior horn proper nucleus neuron axons.
The dorsal cerebellar tract consists of the same half of spinal cord thoracic
nucleus neuron axons.
There is a number of
descending tracts but basic tracts are the anterior pyramidal tract and lateral
pyramidal tract. The pyramidal tracts consist of giant pyramidal neuron axons.
One part of the axons does not cross to form the anterior pyramidal tract. The
other part of them crosses to form the lateral pyramidal tract. The pyramidal
tract nerve fibers end on motor neurons of the spinal cord ventral horns.
CHAPTER 10
THE BRAIN
The Brain consists of
the cerebrum and the brain stem. The latter includes 1) hindbrain (medulla
oblongata and pons), 2) midbrain, 3) and diencephalon. In the brainstem there
are white and grey substances. The white substance is a cluster of nerve
fibers; the grey substance is a mass of nuclei consisting of neurons. In the
brainstem the white substance is embedded in the grey substance. The cerebrum
consists of the cerebral cortex and basal part.
The Brainstem
This includes medulla
oblongata, pons, midbrain, and diencephalon.
Medulla Oblongata
The medulla oblongata includes
transmitter nuclei and cranial nerve nuclei. Transmitter nuclei are 1) nucleus
of fine fascicle, 2) conic nucleus, 3) inferior olive nucleus, 4) additional
medial olive nucleus, 5) additional dorsal olive nucleus, and 6) reticular
formation. Cranial nerve nuclei are 1) nucleus of the hypoglossal nerve, 2)
nucleus of the glossopharyngeal nerve, 3) nucleus of the vagal nerve, 4)
nucleus of the vestibulocochlear nerve, and 5) additional nerve. All
transmitter nuclei consist of associative neurons. The largest is the inferior
olive nucleus. It gives off nerve fibers to the thalamus and cerebellar cortex.
The inferior olive nucleus receives nerve fibers from the red nucleus,
cerebellum, reticular formation, and spinal cord.
In the medulla ventral
part there is the white substance, including myelinated nerve fibers,
constituting the pyramidal tract, traveling from the cerebral cortex. In the
medulla oblongata lateral part nerve fibers pass from the spinal cord, olive
nucleus, and pons to the cerebellum.
Reticular Formation
It is in the upper part
of spinal cord, medulla oblongata, midbrain, and diencephalon. Reticular
formation consists of crossing nerve fibers and small neurons connecting its
various parts. In the reticular substance there are also large neurons
connected with the brainstem nuclei and the spinal cord. Through the reticular
substance the nerve fibers pass from the thin nucleus and conic nucleus to form
a suture. The nerve fibers reach the thalamus. Reticular formation is
responsible for the muscular tonus and stereotype movements.
Pons
On the pons dorsal
surface transverse nerve fibers are seen. On the pons ventral surface the
pyramidal nerve fibers pass. The pons includes their own nuclei, giving off
nerve fibers, conducting nerve impulses to the cerebellum. In the pons there
are cranial nerve nuclei (nuclei of V-VIII cranial nerves). The pons includes
three transmitter nuclei belonging to the cochlear tract.
Midbrain
This includes 1) substance
nigra, 2) tectum, 3) tegmentum of the midbrain, and 4) cerebral crus. Through
the cerebral crus nerve fibers pass from the cerebral cortex to the central
nerve system lower its parts. The neurons of the substance nigra contain the
black pigment. The midbrain tectum includes inferior colliculus and superior
colliculus. In the former there are neurons of the cochlear tract, in the
latter there are neurons of the optical tract. The midbrain tegmentum contains
a number of nuclei. The larges is the red nucleus. Here there are small and
large neurons. The former receive impulses from the cerebellum. Their axons
come in contact with small neurons of reticular formation. Axons of the large
neurons come in contact with the nuclei of the brainstem and spinal cord to
form rubrospinal tract ending on the motor neuron of the spinal cord ventral
horns.
Diencephalon
This is represented by
thalamus, containing a number of nuclei, separated by the white substance from
each other. The thalamus pulvinar neurons come in contact with nerve fibers of
the optic tract. Neurons of the ventral thalamus surface receive impulses from
sensitive nerve fibers (nerve fibers of neurons of the inferior olive, spinal
cord, fine and conic nuclei come in contact with neurons of the thalamus). The
thalamus sends some millions of nerve fibers to the cerebral cortex.
Hypothalamus
The hypothalamus,
localized under the thalamus, regulates all viscera (the stomach, intestine,
spleen etc.), cardiovascular and endocrine systems, sweat and sebaceous glands
of the skin, etc. The hypothalamus is divided into the anterior, middle and
posterior parts. The hypothalamus contains a number of nuclei but the largest
are in the anterior part of the hypothalamus (supraoptic and paraventricular
nuclei).
The supraoptic
nucleus consists of
large cholinergic neurosecretory neurons containing abundant Golgi complex,
rough ER, and mitochondria. In their body and axon there are secretory
granules. The neurons release 2 hormones (vasopressin and oxytocin).
Vasopressin stimulates
the blood vessel muscle contraction and water to reabsorb from the renal
tubules. Oxytocin stimulates the myoepithelial cell contraction in the mammary
gland and smooth muscle cell contraction in the uterine and male sex tracts.
The paraventricular
nucleus includes 2
types of neurosecretory cells: 1) large cholinergic neurosecretory cells and 2)
small adrenergic neurosecretory cells secreting releasing hormones (stimulating
and inhibiting releasing hormones) regulating the hypophysis function.
Middle Hypothalamus Nuclei
It includes 6 nuclei: 1)
arcuate (infundibular) nucleus, 2) ventromedial nucleus, 3) dorsomedial
nucleus, 4) supraoptic nucleus, 5) periventricular grey substance, and 6)
preoptic zone. All these nuclei consist of small adrenergic neurosecretory
cells, secreting releasing hormones.
CEREBELLUM
The cortex covers (Fig.
70A) the cerebellum. The latter consists of the grey substance, including
neurons and glial cells. The cerebellar cortex forms cerebellar gyres and
cerebellar sulci. Profound clusters of neurons form the additional grey
substance of the cerebellum called the cerebellar nuclei. In the cerebellum
there are 1) dentate nucleus, 2) fastigial nucleus, 3) emboliform nucleus, and
4) globose nucleus.
Cerebellar Cortex (Fig. 70A)
It consists of 3 layers:
1) molecular layer, 2) piriform neuronal layer, and 3) granular layer.
Piriform Neuronal Layer
This is a major layer of the cortex.
The piriform cells (Purkinje cells) are efferent associative neurons (from them
efferent nerve fibers begin). These neurons form a row of cells directed across
the gyrus. The Purkinje cell length is about 60 mm. The cell epical part gives off 3-4 branching
dendrites directed into the molecular layer across the gyrus. From the Purkinje
cell basal part the axon arises. It gives off the branches to the adjacent
Purkinje cells. The major branch of the axon passes through the granular layer
and white substance and come in contact with the cerebral nucleus neuron. The
Purkinje cells are responsible for
coordination of movement. All other cortex neurons are auxiliary. They
transmit (conduct) excitation or inhibition impulses to the Purkinje cells.
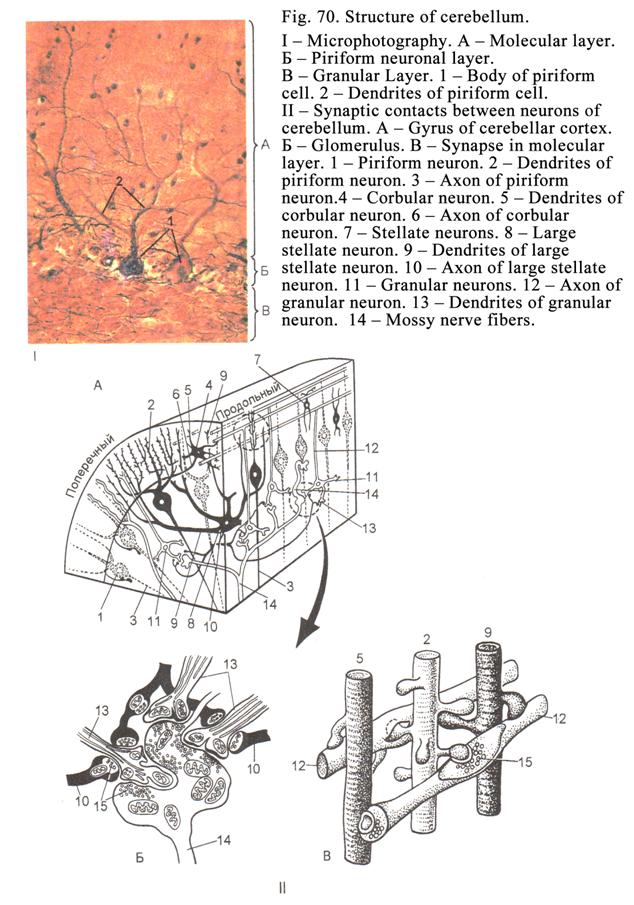
Chapter 10 Brain
Molecular Layer
This contains 2 neuron
types: 1) corbular neurons and 2) stellate neurons divided into large stellate
neurons and small stellate neurons.
Corbular Neurons
These neurons are
localized in the inner one third of the molecular layer. Their dendrites are
branched in the transverse direction of the gyrus. Their axon travels along the
Purkinje cell row and gives off to the cell body branches to form corbular
nerve fibers around every Purkinje cell body. Corbular neurons transmit
inhibition impulses to the Purkinje cells.
Small Stellate Neurons
They are localized in
the external one third of the molecular layer. Their short axon comes in
contact with Purkinje cell dendrites. These cells transmit inhibition impulses
to the Purkinje cell dendrites.
Large Stellate Neurons
These are localized in
the middle one third of the molecular layer. Their dendrites are branched in
the same layer. The branches are directed across the gyrus. Large stellate
neuron axons come in contact with the dendrites or cell body of Purkinje cells.
The large stellate cells transmit inhibition impulses to the Purkinje
(piriform) cells.
Granular Layer
It consists of neurons
of three types (predominently granular neurons).
Granular Neurons
They are the smallest
neurons (5-6 mm in diameter). Almost the entire
cell is occupied by the nucleus. From the cell basal part 3-4 dendrites arise
(similar to a bird paw). The dendrites come in contact with mossy nerve fibers
passing from the pons or inferior olive to form the synapse. The place where
the granular cell dendrites come in contact with mossy nerve fibers is called
glomerulus.
From the granular neuron
apical part the axon arises running to the molecular layer. Here the axon is
divided into 2 branches (bifurcation). Every branch runs along the gyrus and come
in contact with the dendrites of molecular layer neurons (piriform cell
dendrites and others sells). Granular cell axons transmit excitation impulses
to the Purkinje cells (all other cells of the molecular and granular layers
only transmit inhibitory impulses).
Large Stellate Neurons of the Golgi
They are divided into
neurons with long axons and neurons with short axons. The dendrites of large
stellate neurons with short axons run to molecular layer and come in
contact with granular neuron axons while their short axons come in contact with
the granule cell dendrites taking part in cerebellar glomerulus formation and
transmit inhibitory impulses. Large stellate neurons with long axons are
located near the Purkinje cells. Their long axons enter the white matter then
return to the cerebellar cortex and come in contact with its neurons. In the
granular layer there are horizontal neurons. Their dendrites come in
contact with a great number of neurons while their axons come in contact with
neurons of the granular layer of the other part.
Cerebellar Cortex Afferent Nerve
Fibers
Two nerve fiber types enter the
cerebellar cortex: 1) mossy fibers running from the inferior olives and pons
and 2) ascending nerve fibres running from the spinal cord and vestibulocochlear
nuclei of the medulla obligat.
The mossy fibers come in contact with granular
neuron dendrites transmitting the excitant impulses to these cells. The
ascending fibers reach the Purkinje cell dendrites coming in contact with
them to form excitant synapses conducting impulses to piriform neurons.
The Cerebellum Reflex Arc
The arc first neuron is
located in the spinal nerve ganglion (sensitive neuron). The second neuron
(associative neuron) is located in the posterior horn proper nucleus or
thoracic nucleus. The neuron axon, forming ascending nerve fibers, runs to the
dendrite of the Purkinje cell (it is the third neuron). The third neuron axon
(it is initial part of the arc efferent part) conducts nerve impulse to the
fourth neuron located in the cerebellar nucleus. The fifth neuron is a small
neuron of the red nucleus. The sixth neuron is located in the reticular
formation. The seventh neuron is located in the ventral horn motor nucleus. The
seventh neuron axon conducts the impulse to the muscle as a result some muscles
are contracted while others are inhibited. This reflex arc is involuntary. For
example, if a man begins to fall, he cannot understand what happens and his
limbs and trunk move involuntary. After it the man can understand, what
happened, and can comment the case.
The Cerebral Cortex
It forms the gyres and sulci. The
cerebral cortex (Fig. 71A) thickness is about 2-
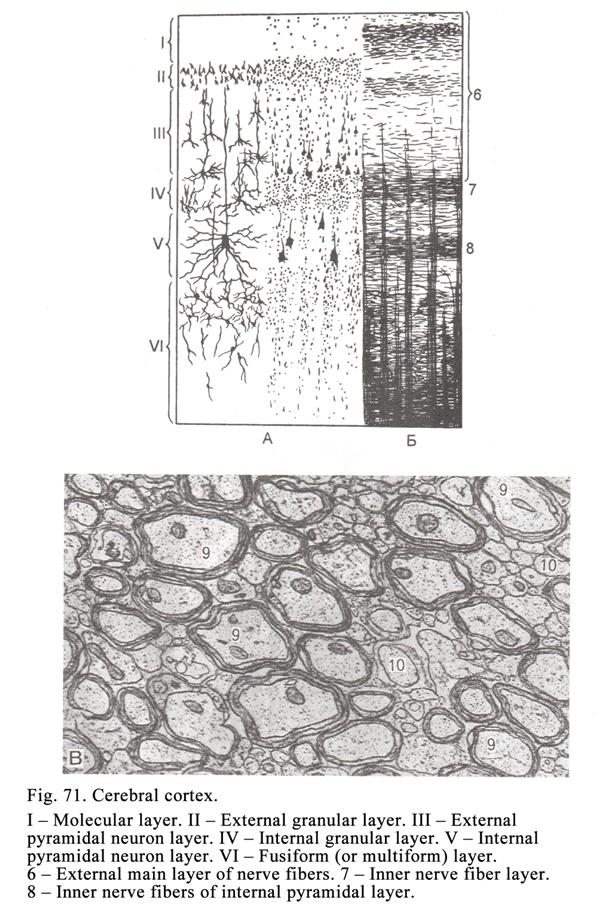
Pyramidal neurons are
10-140 mm in diameter. From the apical part
of the pyramidal neuron the apical dendrite arises reaching the cerebral cortex
molecular layer. From the pyramidal neuron lateral part some lateral dendrites
arise come in contact with adjacent neurons. An axon arises from the pyramidal
neuron basal part.
The cortex development
is more active on the 20th week of the embryo. At this time the
supporting glial cells and glial fibers are formed. The fibers are arranged by
the right angle in relation to surface of future cerebral cortex called
cerebral lamina. At first neurons of the sixth and firth layers enter the
future cortex then neurons of the fifth, fourth, third, and second layers enter
the cortex lamina to form the definitive cerebral cortex. After birth the
supporting glial cells and glial fibers disappear. The arrangement and
structure of the cerebral cortex neurons are called cell architectonic but
arrangement of the cerebral cortex fibers is called fiber architectonic.
The Cerebral Cortex Neurons
Arrangement and Structure
In the cerebral cortex
neurons form 6 weakly limited layers: 1) molecular layer (outermost layer), 2)
external granular layer, 3) external pyramidal neuron layer, 4) internal
granular layer, 5) internal pyramidal neuron layer, and 6) multiform layer
(Fig. 71).
The Molecular Layer
It includes a small amount
of neurons but includes a number of horizontal nerve fibers. The
pyramidal neuron apical
dendrites of the
cerebral cortex enter
its molecular layer. The small spindle shaped neurons are seen there.
Their processes run parallel to the cortex surface.
The External Granular Layer
One consists of small
various shape cells (pyramidal, stellate, oval, and others). The small
pyramidal cell sizes are about 10 mm. The cell apical dendrite arise the molecular
layer; the lateral dendrites are branched in its own layer. The cell axon,
arising from the body basal part, enters the white substance thereafter returns
to the cortex to form cortico-cortical nerve fiber.
The External Pyramidal Neuron Layer
It consists of small and
median pyramidal neurons (10-40 mm). Smaller pyramidal neurons are arranged
upper while larger that are arranged deeper. The apical dendrite of the neuron
reaches the molecular layer and the lateral dendrites come in contact with
adjoining cells. The neuron axon enters the white substance to form
cortico-cortical nerve fiber returning the cerebral cortex and reaching its
molecular layer.
Some of the
cortico-cortical nerve fibers returning into own cerebral cortex hemisphere are
called the associative cortico-cortical nerve fibers. Other cortico-cortical
fibers return to the opposite of the cerebral cortex hemisphere. They are
called the commissural cortico-cortical nerve fibers.
The Internal Granular Layer
This layer consists of
small oval, star shaped, and other neurons. Their dendrites reach the molecular
layer and the axons enter the other layers.
The Internal Pyramidal Neuron Layer
It consists of large
pyramidal neurons (Betzs cells). Betz was the scientist, lived in
The pyramidal neuron
axon branches return to the cerebral cortex, red nuclei, pons nuclei, inferior
olive nuclei, etc.
The Multiform Layer
It contains neurons of
various shapes: fusiform, pyramidal, etc. Their dendrites reach the molecular
layer. Some neuron axons join with pyramidal tract.
The Reflex Arch Passing Through the
Cerebral Cortex
The first neuron is in
the spinal ganglion. The second neuron is found in the proper nucleus of
posterior horn of spinal cord, or the medulla oblongata fine nucleus or cone
nucleus. The third neuron is found in the thalamus. The fourth neuron is found
in the cerebral cortex. The fifth neuron (motor or latter neuron) is found in
the spinal cord ventral horn nucleus. Its axon ends in the muscle fiber of the
skeletal muscle.
The Cerebral Cortex Types
There are two types of
the cerebral cortex: 1) granular type and 2) agranular type.
The Granule Type
It is characterized by
presence of prominent granules layers (II and IV). This cortex type is located
in the sense centers (hearing or visual).
The Agranular Type
It is characterized by
the prominence of other layers (III, V and VI).
The Cerebral Cortex Modules
They are represented by
macro columns, which are 300 mm in
diameter (Fig. 72). The module is a repeating (a number of times) structure
providing the same function. In the human cerebral cortex there are 3 millions
modules. Every column (module) rounds a cortex-cortical nerve fiber (cerebral
cortex pyramidal neuron axon of the second or third layers). The macro column
consists of some micro columns. The latter are less than 100 mm in diameter. Each module is
connected with 3 modules their own cerebral cortex hemisphere and 2 modules of
the second hemisphere.
Every module includes
the excitement and inhibition systems.
The System of Excitement
It consists of nerve
fibers and neurons. Every column is provided with special nerve fibers (arising
from thalamus) ending on the spinal star shaped neurons of an internal granular
layer or the pyramidal neuron dendrites of the cerebral cortex external
pyramidal layer (III layer). Thus, the spinal star shaped neurons and II &
III layer pyramidal neurons belong to the system of excitant. There are 2 types
the spinal star shaped neurons: 1) neurons which axon endings end on the
pyramidal neuron epical dendrite and 2) diffuse type neurons which axon endings
end on the pyramidal neuron basal dendrite. The pyramidal neuron axons are
cortico-cortical nerve fibers.
From the pyramidal neurons of every module
(column) 3 the cortico-cortical nerve fibers move off. They enter the white
substance then come back to their own cerebral hemisphere. These fibers are
called the associative cortico-cortical nerve fibers. Round every
cortico-cortical fiber macro column is formed. In addition from every module 2
cortico-cortical nerve fibers move out, they pass into other (apposite)
hemisphere trough the corpus callosum. These fibers are called the commissural
cortico-cortical nerve fibers. Round these fibers the macro column is formed as
well.
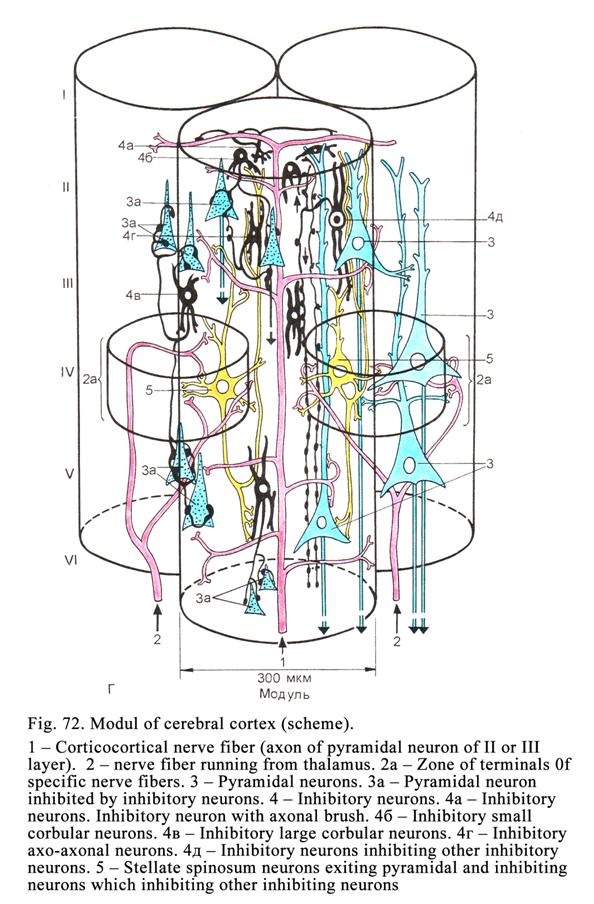
Thus, every module is
connected with 3 other modules of their own hemisphere and 2 modules of
opposite hemisphere.
The cortico-cortical
nerve fiber passes from the white matter and cortex multiform layer to the
cortex molecular layer. Its branches come in contact with neurons every
cortical layer. Reached the molecular layer the cortico-cortical nerve fiber is
divided into 2 branches spreading further to the column.
Thus, the system of
excitement includes two special nerve fibers arising from a thalamus, two types
of spinal star shaped neurons, pyramidal neurons, and cortico-cortical nerve
fibers.
The System of Inhibition
It includes 4 types of
inhibiting neurons: 1) neuron with axonic brush, 2) corbular large and small neurons,
3) axoaxonal neuron, and 4) neuron with two clusters of dendrites.
The inhibiting
neurons with axonic brush are located in a molecular layer. They produce inhibition synapses in
the branches of cortico-cortical nerve fibers and prevent spreading of the
nerve impulses.
The small corbular
neurons are located
in the II, III, and V layers. They produce inhibition synapses with the
pyramidal neurons of those layers.
The large corbular
neurons are also
located in II, III, and V layers. They produce the inhibiting synapses on the
pyramidal neurons of those layers but outside the macro-column.
The axon-axonic
inhibiting neurons
are lodged in II and III layers. They form the inhibiting synapses on the
pyramidal neurons of the second and third layers.
The neurons having
two clusters of dendrites produce inhibiting synapses on the rest inhibiting neurons. Then the
pyramidal neurons are not inhibited. The neuron, having two clusters, receives
the nerve impulses from the spinal star shaped neurons. The pyramidal neurons
also receive the impulses from the spinal star shaped neurons therefore the
pyramidal neurons are not inhibited but are excited at the same time.
Arrangement of Cerebral Cortex
Fibers
In the cerebral cortex
there are associative nerve fibers, joining its own hemisphere parts, and
commissural nerve fibers, connecting right and left hemispheres, fibers,
connecting the cerebral cortex with lower central nerve system parts, and the
horizontal nerve fibers (localized in molecular layer, internal granular layer,
and internal pyramidal neuron layer).
Meninges
The brain and spinal
cord are covered by 3 meninges: 1) pia mater, 2) arachnoid mater, and 3) dura
mater.
The Pia Mater
It consists of loose
connective tissue containing blood vessels, nerve fibers, and single neurons
closely joined with brain substance.
The Arachnoid Mater
It is stretched between
gyres. Between the pia mater and arachnoid mater there are subarachnoid space
filled with the cerebrospinal fluid. Collagen fibers run from the pia mater to
the arachnoid mater.
The Dura Mater
It is connected with a
periosteum. The dura mater consists of a dense connective tissue. Between the
dura mater and arachnoid mater there is the subdural space filled with fluid.
In the spinal cord between a periosteum and spinal dura mater there is the
epidural space filled with connective tissue.
Blood Supply of the Brain and Spinal
Cord
Thevenralroot arterie
and dorsal root
arteries enter the
spinal cord and
branch in a pia mater. The artery
small branches enter the white and grey matters. A large artery runs along
ventral median fissure. Veins are located in the dorsal part of the spinal cord
and in the pia mater. Deoxygenated blood drains from the spinal cord through
the ventral root veins and dorsal root veins.
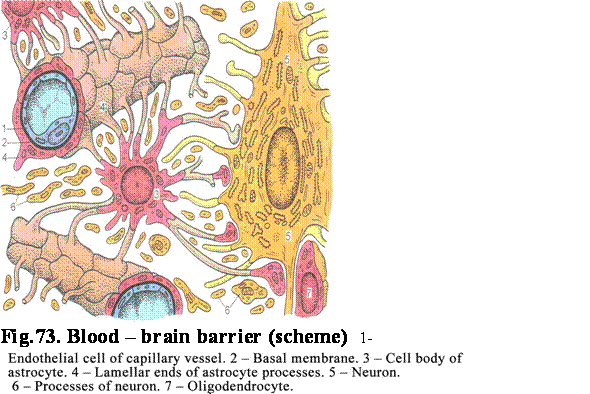
The brain vessels are
formed from carotid and vertebral arteries, which make an anastomose forming
the basal artery. The artery branches into the cranial pia mater small arteries,
entering the grey and white substances of the brain. The brain blood capillary
vessels have continues endothelial lining and in addition continuous basal
lamina (membrane) around the capillary. The astrocyte processes cover the
capillary wall surface, as a result the encephalon blood barrier is formed
(Fig. 73) possessing of selective permeability.
The Autonomic Nervous System
It innervates viscera,
endocrine and exocrine glands, blood and lymphatic vessels. The autonomic
nervous system is divided into the sympathetic and parasympathetic. Both
sympathetic and parasympathetic nerve systems include central and peripheral
parts.
The Sympathetic Nervous System
Central part of the
sympathetic nerve system is located in the lateral nucleus of the grey commissure
of the spinal cord, spreading the firth thoracic segment to the second lumbar
segment. The sympathetic nervous system peripheral part is localized in the
sympathetic ganglions sitting in left and right trunks near the vertebral
column, and the prevertebral sympathetic ganglions forming the abdominal
plexus.
The Sympathetic
Ganglion Structure. The
sympathetic ganglion includes two types of neurons: 1) efferent neurons and 2)
small intensive fluorescence neurons (inhibiting neurons). The satellite cells
and thin connective tissue layer surround every neuron. Entire ganglion is
covered by connective tissue capsule. The efferent multipolar neurons receive
impulses from preganglionic myelinated cholinergic nerve fibers (neuron axons
from the lateral nucleus of the grey commissure of the spinal cord). The
efferent neuron axons, forming postganglionic nonmyelinated adrenergic nerve
fibers, end on effectors (smooth muscle or glands). The small intensive
fluorescence neurons also receive impulses from preganglionic myelinated
cholinergic nerve fibers. Bodies and axons of these neurons contain
noradrenalin. These neuron (inhibiting neuron) axon terminals contain
noradrenalin as well. When nerve impulse reaches the terminals, the
noradrenalin releases from them and travels to the efferent neurons to inhibit
the latter.
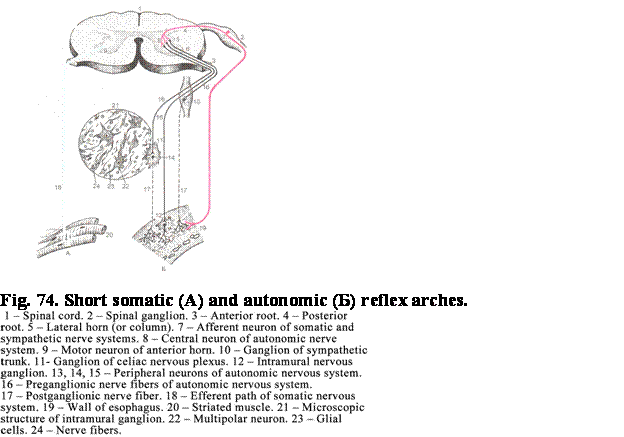
The Sympathetic Nervous System
Reflex Arc
The simplest sympathetic
reflex arc (Fig. 74Į)
includes 3 neurons: 1) the spinal ganglion sensitive neuron, 2) associative
efferent neuron of the grey commissure lateral nucleus of the spinal cord, and
3) efferent neuron of the sympathetic ganglion (Fig. 74).
The Parasympathetic Nervous System
(Fig. 74Į)
The central part of the
parasympathetic nervous system includes the III, VII, IX, and X pairs of
cranial nerves and pelvic part of the lateral nucleus of the grey commissure of
the spinal cord.
The Intramural (Parasympathetic)
Nervous Ganglion Structure
These ganglia are
localized near or within the viscera. They are covered with connective tissue
capsule from which the connective tissue intends to form the ganglion stroma.
The ganglion includes 3 neuron types: 1) efferent neuron (the Dogels neuron I
type), 2) sensitive neuron (the Dogels neuron II type), and 3) associational
neuron (the Dogels neuron III type) connecting two adjoining ganglions. Around
the body of every neuron there are glial cells (satellite cells), forming the
glial capsule, covered by thin connective tissue capsule. Thus, both glial and
connective tissue capsule cover each neuron body. In the ganglion there are
glial macrophages.
I type of Dogels
neurons are
multipolar efferent cells. They receive impulses from nerve fibers moving from
the central part of parasympathetic nervous system and from sensitive neuron of
parasympathetic ganglion. The efferent neuron axon runs from cell body as
postganglionic nonmyelinated cholinergic nerve fiber ending on the smooth
muscle or gland.
II type of Dogels
neurons is also
multipolar neurons having axon and number dendrites of the equal length. The
neuron dendrites end with receptors, but the neuron axon contacts with the
efferent neuron.
III type of Dogels
neuron has some
dendrites and long axon reaching the neuron of adjoining ganglion.
The Parasympathetic Nervous System
Reflex Arc
The reflex arc (Fig. 74)
can consist of 3 or 4 neurons. The first (sensitive) neuron of the reflex arc,
containing 3 neurons, is located in the spinal ganglion or ganglions of the
III, VII, IX, and X cranial nerves. The second neuron (the associative efferent
neuron) is found in the spinal cord or nuclei of the III, VII, IX, and X
cranial nerves. The third (efferent) neuron
is the Dogels
neuron I type. The axon of this neuron forms
postganglionic nonmyelinated cholinergic nerve fiber coming in contacts with
smooth muscle or glands.
The reflex arc,
including 4 neurons, has additional neuron, i.e. the second type of Dogels
neuron.
The Parasympathetic Local Reflex Arc
The local reflex arc
includes only 2 neurons: 1) the first neuron is the sensitive neuron (the
Dogels neuron II type) and 2) the second neuron is the efferent neuron (the
Dogels neuron I type).
Distinctive Features of Alimentary
Tract Nervous Ganglia
In digestive tract wall
there are 3 nervous plexuses: 1) subserous, 2) intramuscular, and 3)
submucosal. The larges of nervous plexus is intramuscular one. Its ganglions
include not only efferent neurons (the Dogels neuron all types) but also
adrenergic neurons containing catecholamines. When the adrenergic neurons are
excited, their axons release catecholamines, which move to other neurons and
inhibit them. These neurons of ganglion can contain purin (neurotransmitter).
In addition these ganglions have cells, releasing VIP, substance P, serotonin,
histamine, and many others hormones.
The nervous system
provides integrative, adaptive, and regulative functions.
CHAPTER 11
SENSORY ORGANS & EYE AND OLFACTORY ORGAN
The sensory organs are
peripheral parts of reflex arcs include: 1) sensitive neurons of sensory organ,
2) associative afferent neurons conducting a nerve impulse, and 3) the cerebral
cortex neurons providing analysis and synthesis of the received impulse to make
a response.
Classification of the Sensory Organs
The sensory organs are
classified into 3 types: 1) organ of vision and olfactory organ, 2)
vestibular-cochlear organ and gustatory organ, 3) receptors scattered in whole
body.
I Type Organs
They are characterized that
their sensitive cells are neurons (nerve sensory cells) deriving from the
nervous sources.
The II Type Organs
The sensory cells,
called sensory epithelial cells, of these organs are derived from ectoderm. The
sensory epithelial cells transmit the impulse to the neuron cells called the
secondary nerve sensitive cells. Special sensitive hairs or stereocilia are
located in the apical part of the sensory epithelial cells.
Organ of Vision
It is represented by
eyeball, localized in orbital cavity, and accessory visual apparatus including
eyelids, lacrimal apparatus, and external ocular muscles.
The Eyeball
In the eyeball there are
3 tunics. The outermost tunic is fibrous tunic consisting of 2 parts: 1)
anterior part (cornea) and 2) posterior part (sclera). The middle tunic is
vascular tunic (uvea). The innermost tunic is retina.
The eyeball includes 3
apparatuses: 1) dioptric apparatus (cornea, aqueous humor of posterior and
anterior chambers, lens, and vitreous body), 2) accommodative apparatus
consisting of ciliary body and ciliary suspensory ligament (some authors refer
an iris to this apparatus but in fact it belongs to adaptive apparatus), and 3)
light receptive apparatus (retina).
The Eyeball Formation
The vision organ is developed as
outgrowth from the brain (Fig. 75). The proximal part of the outgrowth remains
narrow and is called the optic stalk. The distal part of the outgrowth forms a
rounded hollow structure called the optic vesicle.
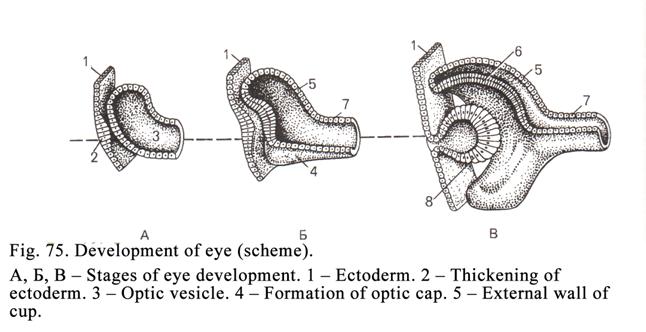
This vesicle is
invaginated by surrounding tissues so that it is converted into a two layers of
an optic cup. The outer layer of the cap persists as single layered epithelium,
which becomes pigmented. It forms the pigment cell layer of the retina. The
cells of the inner layer multiply to form several layers of cells, which become
the nervous layer of the retina. The cells of the skirt of the optic
cap multiply to
form sphincter of pupilla and dilator of pupilla. The connective tissue
of the sclera, iris, and ciliary body are derived from mesenchyme.
The ectoderm apposite
the optic cap invaginates into latter to form a vesicle. The vesicle separates
from ectoderm to form lens (Fig. 75).
The Sclera
The sclera consists of
the collagen fibrous tissue. Some elastic fibers and connective tissue cells
(mainly fibroblasts) are also present. Its thickness is about
The sclera along with
the cornea collectively forms the fibrous tunic of the eyeball. The sclera
protects delicate structures within the eye, resists intra ocular pressure, and
maintains the eyeball shape. It also provides an attachment to the muscles
moving the eyeball.
The Eyeball Dioptric Apparatus
The apparatus includes
the cornea, aqueous humor of anterior and posterior chambers, lens, and
vitreous body.
The Cornea (Fig. 76, Fig. 77)
The cornea is of convex-concave
shape, i.e. it converges the light beams. Its refractive index is 1.37. The
cornea is made up of 5 layers: 1) the outermost layer (anterior epithelium), 2)
anterior limiting lamina, 3) proper substance, 4) posterior limiting lamina,
and 5) corneal endothelium.
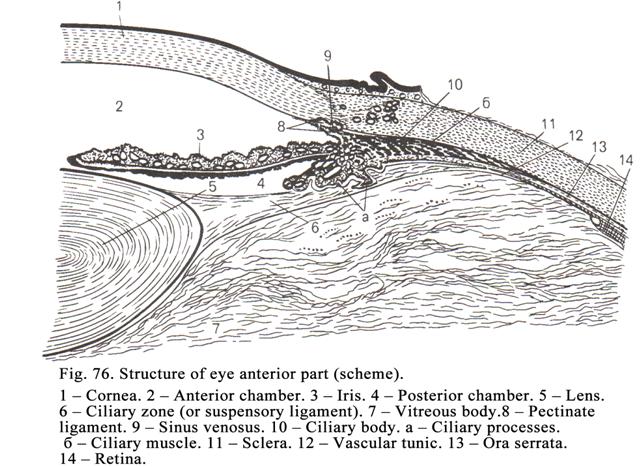
The outermost layer is the stratified squamous
nonkeratinized epithelium. The cells in the deepest layer of the epithelium are
columnar. In the middle layer they are polygonal. In the superficial layer they
are flattened. The epithelium lies on the basal membrane consisting of two
layers (external and internal).
The anterior limiting
membrane (Bowmans membrane) made up of fine collagen fibrils embedded in the amorphous matrix. Its
thickness is 6-10 mm.
The proper substance is composed by connective tissue
lamellae consisting of parallel-arranged collagen fibers. Each lamella is made
up of 1000 collagen fibers, which are of 0.3-0.6 mm in diameter. Between the lamellae there are
fibroblasts and ground substance consisting of large amount of sulfated
glycosaminoglycans.
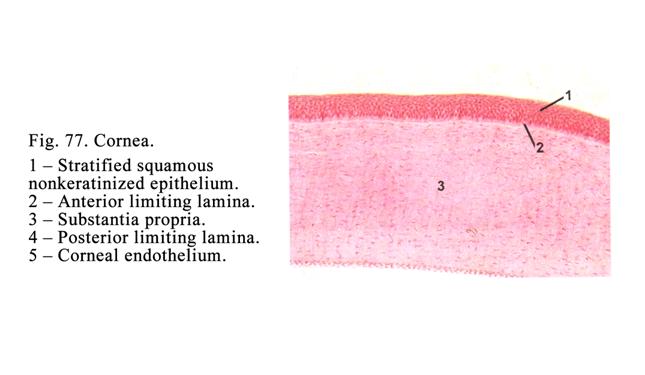
The cornea is
transparent as it consists of sulfated glycosaminoglycans and blood vessels are
absent in it. The nutrition of the cornea is provided by the sclera blood
vessels and anterior chamber fluid.
The posterior
limiting lamina
consists of the amorphous matrix and thin collagen fibrils. It is 10 mm in thickness.
The corneal
endothelium layer consists
of one row flattened epithelial cells.
The Iridocorneal Angle
It is formed by the iris
and cornea (Fig. 76). In the cornea (opposite the iridocorneal angle) there is
the groove (sulcus). The sulcus posterior edge is swollen. It is made up of
circular collagen fibers. At this place the iris and ciliary body are attached
to the sclera (attaching apparatus). The attaching apparatus consists of the
trabecular part and pectinate ligament. The trabecular part is made up of the
trabeculae. The trabecula is band shaped. The trabecula central part contains
the collagen fiber. Elastic fibers and hyaline mass surround the fiber. Endothelial cells cover each trabecula.
Liquid enter the sinus venosus of sclera through slit spaces between the
trabeculae from the eye chambers.
The sinus venosus of the
sclera is the slit like space or some splits (width 2-
The Lens
It is located behind the
anterior chamber (Fig. 76. 5) in the ciliary body ring center. The lens is
fixed with the ciliary suspensory ligament. The lens lodges in the highly
elastic transparent capsule (11-18 mm in thickness). The collagen fibers of the
ciliary suspensory ligament are attached to the capsule edges. Deep to the
capsule the anterior lens surface is covered with simple squamous epithelium.
However, in the periphery of the lens the epithelial cells become progressively
longer. The epithelium of the lens edge becomes columnar. It acquires the
ability to divide with mitosis (growth zone of lens). From the lens edge the
epithelium spreads to the posterior surface of the lens. The lens posterior
surface epithelial cells convert into lens fibers (principal, transitory, and
central fibers). The central fibers form nucleus of the lens (firm substance of
the lens central part). The outer part of the lens is pliable. At first the
lens fiber includes the nucleus and cytoplasm. The cytoplasm consists of the
special transparent proteins. The lens fibers are adhered with each other
special substance with the same refractive index, which is similar to that of
lens fiber transparent proteins (1.42). The lens is a pliable structure
therefore it constantly strives to become spherical. The ciliary suspensory
ligament, stretching out the lens, makes it flat.
Vitreus body
It is located behind the
lens. The vitreous body consists of jelly like substance (vitreous protein) and
thin collagen fibrils forming the net. The vitreous body peripheral part is
denser than the central part, which is called the visual canal passing to the macula
lutea (this is the area of the clearest vision). The refractive index of the
vitreous body is 1.33.
The dioptric apparatus
function is to transmit the light beams to the macula lutea.
The Accommodative Apparatus
It consists of the
ciliary body and the ciliary suspensory ligament (some authors refer the iris
to the accommodative apparatus)
The Ciliary Body (Fig. 76)
This is a ring-like
structure. In the section its rim is triangular. The triangle basal part is
directed to the front but the sharp angle is directed to the back. The ciliary
body can be divided into the external plat part, called the ciliary ring, and
internal part called the ciliary corona. The ciliary body is covered by the
epithelium passing from the retina. The epithelium consists of the basal
pigmented cuboidal cells and superficial non-pigmented columnar cells. The
epithelium is covered by ciliary membrane. The epithelium releases aqueous
humor. The ciliary corona gives off ciliary processes. Each process has a core
of connective tissue and blood vessels.
The ciliary body does
not include only epithelial and connective tissue but also muscle tissue
(ciliary muscle). The latter consists of longitudinal (meridian muscle fibers),
radial, and circular muscle fibers forming 2 layers: 1) external layer,
consisting of the longitudinal muscle fibers, and 2) internal layer consisting
of the radial and circular muscle fibers.
The Ciliary Suspensory Ligament
(Fig. 76)
It consists of the
radial collagen fibers. The external ends of the fibers attach to the ciliary
body processes while their internal ends attach to lens capsule. Thus, the lens
is fixed in the ciliary body ring center.
The ciliary body is
responsible for production alteration in the convexity of the lens (through the
ciliary body suspensory ligament) enabling the eye to see at varying distances.
It is called the accommodation.
The Alteration of Accommodative
Apparatus Depends on Distance between the Eye and Object
In order to the eye to see
the near object the ciliary body muscle must contract to make the ciliary body
diameter less as a result the suspensory ligament collagen fiber tensions
become weak therefore the lens becomes more convex and the focus distance of
lens becomes shorter.
To see the far object
the ciliary body muscle must relax to make the ciliary body diameter larger,
the tension of the suspensory ligament collagen fibers becomes stronger
therefore the lens convex becomes less and focus distance of lens becomes
longer.
Thus, if the distance
between the eye and object is short (reading book) the ciliary body muscle
contracts and the eye becomes quickly tired.
The vascular tunic
(uvea) includes
the choroid, ciliary body, and iris.
The Choroid
It includes 4 layers: 1)
suprachoroidal lamina, consisting of connective tissue, containing a number of
pigment cells, 2) vascular layer, consisting of the small arteries and veins,
interspace between them is filled with loose connective tissue, containing
abundant number of melanocytes. 3) choroid capillary lamina formed by
capillaries, arising from vessels of the second layer, and without uniform
diameter in their length, and 4) basal complex consisting of collagen layer,
elastic layer, and basal lamina (membrane) applied to the retinal pigmented
layer. The entire basal complex thickness is about 4 mm. The choroid provides nutrition of
the retina.
The Adaptive Apparatus
It is the part of the
accommodative apparatus represented by the iris and pigmented layer of the
retina.
The Iris (Fig.76, Fig. 78)
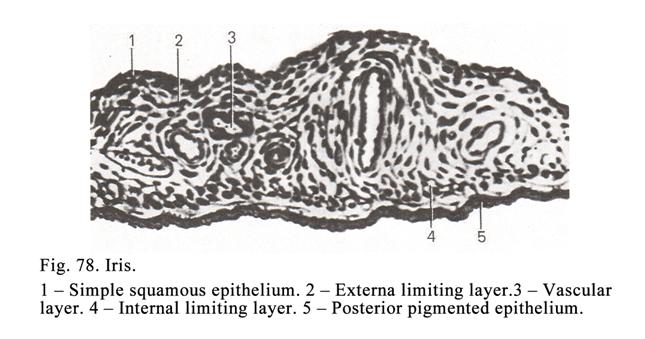
It has a discoid form.
The aperture (pupil) is in the iris center. The iris periphery is continuous
with the ciliary body. The iris is made up of 5 layers: 1) anterior epithelial
(endothelial) layer, 2) anterior (external) limiting layer, 3) vascular layer,
4) posterior (internal) limiting layer, and 5) pigmented epithelial layer.
The anterior
epithelial layer is
represented by flat endothelial cells, which continuous with the posterior
endothelial layer of the cornea.
The anterior limiting
layer is
characterized that it contains loose connective tissue including numerous
melanocytes. The eye has various
colours. It depends
on amount and kind of melanocytes and pigments. If in the iris layer
pigment is not, the eye is red as the eye tissues are relatively transparent
(translucent) and the colour of blood is seen.
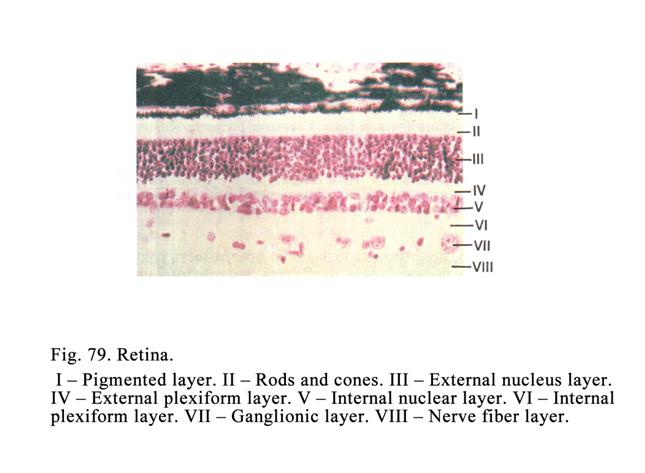
The vascular layer is the plexus of small arteries and
veins. Loose connective tissue with pigment cells is between them.
The posterior
limiting layer consists
of loose connective tissue containing melanocytes. This layer also includes
smooth muscle fibers arranged circularly (sphincter pupilla muscle) and smooth
muscle fibers arranged radially direction (dilator pupilla muscle). The
sphincter pupilla muscle is innervated
by ciliary nerve
anglion while dilator pupilla muscle
is innervated by cervical superior sympathetic ganglion.
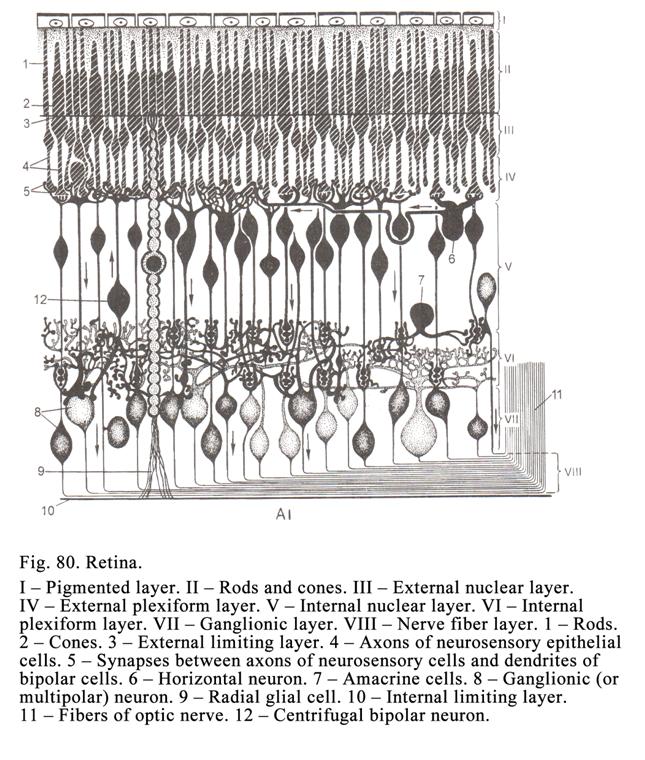
The pigmented
epithelium of iris
consists of two layers.
Cuboidal-pigmented epithelium lies in the basal membrane. The
superficial layer is columnar non-pigmented epithelium.
The iris pupil regulates
the light amount passing into the eye. In bright light the pupil contracts
while in dim light it dilates so that the optimum amount of light required for
proper vision reaches the retina.
The Retina
It is innermost tunic of
the eye. The retina is light sensitive structure (light receptive apparatus).
The retina part near the ciliary body is not light sensitive (part caeca
retinae) but its posterior part is light sensitive.
The sensitive part of
the retina (Fig. 79, Fig. 80) includes the pigment layer and nervous layer
consisting of 9 layers so the retina includes 10 layers (1 pigment layer + 9
nervous layers). The nervous layer is a chain consisting of 3 neuron types: 1) neurosensory
epithelial cells, 2) associative neurons (bipolar, horizontal, and amocrine
neurons), and 3) ganglionic neurons.
The cell neuron bodies
form 3 layers: bodies of neurosensory cells constitute the external nuclear
layer, associative cell bodies constitute the internal nuclear layer, and
ganglionic neuron bodies constitute the ganglionic layer.
The processes of the
neuron cells form more 4 layers: the rod and cones of the neurosensory cells form
layer of rods and cones, the axons of the neurosensory cells contact with
dendrites of associative cells to form the external plexiform layer, the axons
of associative cells contact with dendrites of the ganglionic cells to form the
internal plexiform layer, the axons of the ganglionic cells form nerve fiber
layer.
Thus, the bodies of the
nervous cells form 3 layers and their processes form 4 layers so 7 layers are
formed. But where are more 3 layers? The eight is the pigment layer. But where
are 2
other layers? The retina contains
radial glial cells. Their peripheral
processes interlace to form external limiting layer their internal processes
interlace to form internal limiting layer.
Thus, the pigment retina
cells, bodies of neurons, their processes, and that of radial glial cells make
10 layers: 1) pigmented layer, 2) rods and cones layer, 3) external limiting
layer, 4) external nuclear layer, 5) external plexiform layer, 6) internal
nuclear layer, 7) internal plexiform layer, 8) ganglionic layer, 9) nerve
fibers layer, and 10) internal limiting layer.
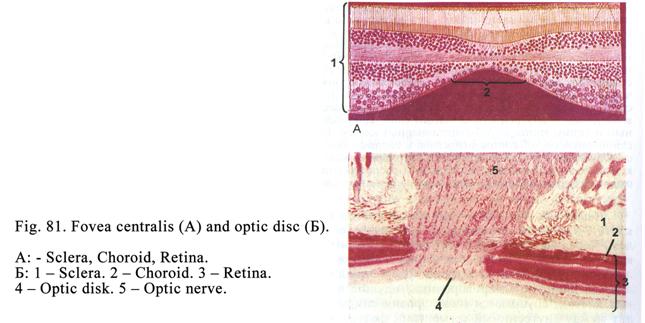
In human eye the
neurosensory cell receptors (rods and cones) direct to the retina pigment, i.e.
the light beams have to pass from the tenth to the thord layer (internal
limiting layer, nerve fiber layer, ganglionic layer, internal plexiform layer,
internal nuclear layer, external plexiform layer, external nuclear layer,
external limiting layer and at last they reach the rods and cones).
The retina macula lutea
is the region of the clearest vision. In the macula lutea center there is the
central area. In the central area all layers (except external nuclear layer
consisting of neurosensory cone cells) are thinned (Fig. 81A). A short distance
to the macula lutea there is optic disc. It is formed by ganglionic neuron
axons constituting the nerve fiber layer. The optic disc (Fig. 81Į) is an initial part of the optic
nerve. Thus, the optic nerve is formed
by ganglionic neuron axons.
The Structure of the Neurosensory
Epithelial Cells
The neurosensory epithelial
sells are rod cells and cone cells.
Neurosensory Rod Epithelial Cells
(Fig. 82)
Their bodies are located
in the external nuclear layer. Each cell consists of cell body, one axon and
one dendrite. The nucleus is in the body center. The area round the nucleus is
called the perikaryon. From the perikaryon the central process, called the
axon, rises. It comes in contact with dendrites of interneurons. The peripheral
process (dendrite) end is called rod.
The rod consists of 2
segments: external segment and internal segment. The external segment is
made up of membranous discs (their amount is about 1000). Every disc is
composed of 2 membranes (double membrane). The disc thickness is 15 nm; its
diameter is 2 mm.
Distance between the discs is 15 nm. Distance between own disc membranes is 1
nm. The discs are formed as follows. The external segment cell membrane basal
part invaginates into the segment to form the long fold (2 mm). After that the fold separates
from the cell membrane to form disc.
The rod external segment
contains photosensitive pigment (visual purple or rhodopsin) that is concerned
with the conversion of light into nerve impulses. The rhodopsin is composed of
the protein and the aldehyde of the vitamin A. Thus, vitamin A is necessary for
the rod active function.
The external segment is
joined with the internal segment by cilium consisting of 9 pairs of the
peripheral tubules and 1 pair of the central tubules. The microtubules are
attached to the basal body.
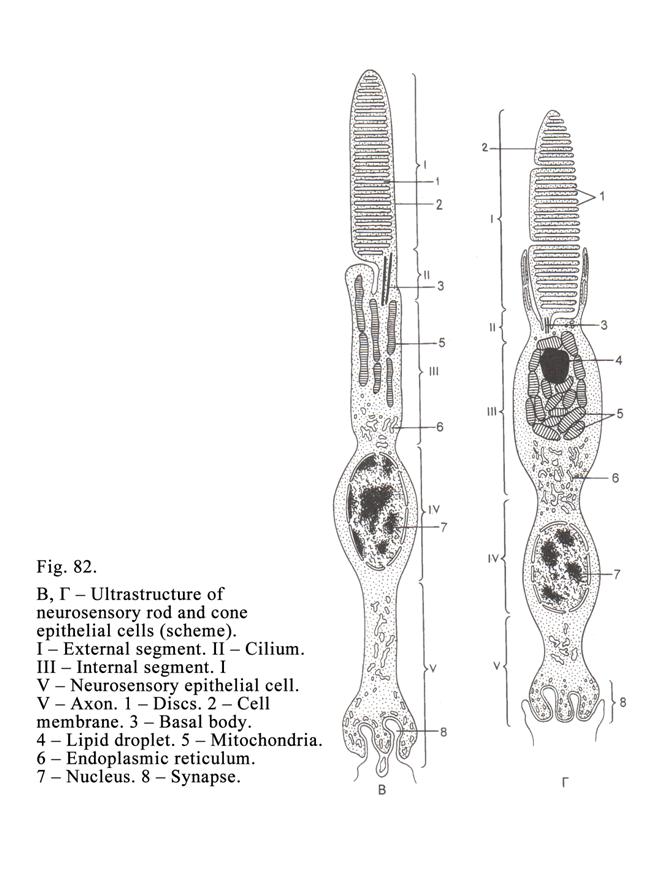
Within the internal
segment there are
enzymes and organelles. The rods are responsible for receiving poor light. The
amount of the rod epithelial cells in the man eye is about 130 millions. The
length of largest rods is about 75 mm.
Neurosensory Cone Epithelial Cells
(Fig.82)
They consist of the
perikaryon, axon and dendrite (peripheral process). The axon comes in contact
with bipolar neurons of the retina. Their dendrite is ended by the
photoreceptor called the cone. The structure, shape, and photosensitive pigment
of cones differ from those of rods. The pigment is called iodopsin.
The cone external
segment includes about 1000 cemidiscs. The cemidisc is formed by the
invagination of the external segment cell membrane into the latter but it is
not separated from the cell membrane, i.e. it is joined with the cell membrane
of the external segment. The latter is joined internal segment by a cilium.
The internal segment
contains organelles and ellipsoid consisting of lipid droplet surrounded by
mitochondria. The ellipsoid is believed to play certain role in respond to
bright light.
The amount of the
neurosensory cone epithelial cells is 6-7 millions. They respond to bright
light. Cones are believed to be of three types: red sensitive, blue sensitive,
and green sensitive. Owing to the combination of the cones three types the man
eye can receives all the colors of a rainbow. The presence or absence of one or
another pigment within the cones depends on the presence or absence of a
corresponding gene within the X sex chromosome.
If the cones, containing
the red pigment, are absent, the person cannot see the red light (it is called
prtanopia), if the cones, containing green sensitive pigment, are absent, the
person cannot see the green light (it is called deuteranopia).
Associative neurons
The associative neurons
are bipolar, horizontal, and amacrine neurons.
The bipolar neuron bodies are located in the internal
nuclear layer. They give off dendrites that enter the external plexiform layer
to form synapses with some of the neurosensory rod cell axons or one of the
neurosensory cone cell axon. The bipolar cell axons enter the internal
plexiform layer to form synapse with dendrites of multipolar ganglionic
neurons. The bipolar neurons transmit visual impulses from neurosensory
epithelial cells to ganglionic neurons.
The horizontal neuron bodies are located in the internal
nuclear layer near the neurosensory epithelial cells. Their dendrites contact
with the axons of the neurosensory epithelial cells locating in the retina
center. Their long axons also contact with those located in the periphery of a
retina to form inhibiting synapses (it is called lateral inhibition providing a
clear image of object on the retina).
The amacrine neuron bodies, lying in the internal
nuclear layer, contact with ganglion neurons. They provide function similar to
the horizontal neurons in relation to the ganglionic neurons.
Multipolar (Ganglionic) Neurons
They are located in the
ganglionic layer. Their dendrites come in contact with the bipolar and amacrine
neurons while the axons constitute the nerve fiber layer. The nerve fibers of
the nerve fiber layer run to an optic disc to form the optic nerve.
The Optic Pathway
This is chain (of
neurons) consisting of 5 neurons. The first neuron type is neurosensory epithelial
cell. The light affects the photoreceptive pigment (rhodopsin or iodopsin). It
leads to the alteration of the permeability of the photoreceptive neuron cell
membrane as a result, nerve impulses are formed. The impulses are transmitted
to the bipolar neuron (second neuron type) then to the multipolar neuron. The
multipolar neuron axons constitute the optic nerve entering the cranial cavity.
Within the latter the multipolar neuron axons, included into the optic nerve,
reach the optic chiasm. Here the axons of the internal halves of the optic
nerves (right and left) cross to form right and left optic tracts. Each optic
tract contains the axons of multipolar (ganglionic) neurons from the right and
left eyes.
The axons, included into
the optic tracts, reach the fourth neurons located in the thalamus pulvinar,
the lateral geniculate body and the superior colliculus. The axons of the
fourth neurons, locating in the pulvinar and lateral geniculate body reach the
calcarine sulcus (sensitive optic center) of the cerebral cortex where the next
(fifth) neuron is located.
The Retina Pigmented Layer
The pigmented layer
consists of 6 millions of pigment cells, lying on the vascular tunic basal
membrane. The light cytoplasm of the pigment cells, with a small amount of
organelles, contains a number of pigment granules. The nuclei of the cells are
round shaped. The outgrowths (microvilli) rise from the apical surface of the
pigment cells. The latter enter between the rods and cones. Every rod is
surrounded by 6-7 microvilli, while each cone is surrounded by 40 microvilli.
The pigment granules can move from the cell body to these outgrowths and from
outgrowths in the cell body. The movement is regulated by the melanocytes
stimulating hormone (MSH) of the hypophysis cerebry pars intermedia. Intracellular filaments also take part in
this process. The pigmented layer provides many functions: 1) it is a part of
the eye adapting apparatus; 2) it inhibits the peroxidization, 3) it is concerned
with phagocytosis, and 4) it takes part in metabolism of the vitamin A.
Participation in Adaptive Function
With the bright light
the rods and cones receive too many light beams. The pupil shrinks rapidly to
decrease the light amount. But there is filling of discomfort in the eye. At
the same time the cell pigment moves from the body to the outgrowths, located
between the rods and cones, as a result the pigment beard is formed. Since the
rods are not sensitive to the bright light they become longer and enter the
pigmented beard. At the same time the cones become shorter and repel from the
pigment beard to get much light. Thus, the pigmented bread like the screen
protects the rods from bright light. At the same time there is no discomfort in
the eye.
In the dim light the pupil rapidly
dilates however the eye cannot see the objects well. But in some minutes the
eye sees the objects well. At the same time pigment leaves the outgrowths of
the pigment cells, i.e. the pigment bread disappears or becomes shorter. Since
the cones cannot get the dim light they become longer and enter the short
pigment beard while the rods become shorter and repel from the pigment beard to
receive more light.
Participation in the Peroxidization
The inhibition of the
peroxidization is provided by two ways: 1) the pigment cell peroxisomes release
the enzyme catalasa inhibiting the peroxidization and 2) the pigment granule
surface absorbs molecules of metals catalyzing the peroxidization.
Participation in the Vitamin A
Metabolism
The vitamin A stores in
the liver. Special protein, synthesized in the liver, carries the vitamin A to
the retina pigment layer. Vitamin A molecules, taken by pigment cells
receptors, enter the cell body, where rhodopsin is synthesized, which enters
the discs of the rods.
Participation in the Phagocytosis
The pigment cells engulf
about 80 discs of rods or cones per every day.
Regeneration of the Rods and Cones
Regeneration of the rods
and cones is provided as follows. Firstly the external segment of the apical
part discs (of rods or cones) become degenerative while basal part of the
external segment cell membrane is elongated and invaginates into the segment to form a new
disc. At the same time the generative discs are engulfed by pigment cells. Thus,
every day 80 discs in each external segment are engulfed and 80 new discs are
formed so all discs of external segments are renewed in 12 days.
The process of the
formation of the new discs and their engulfing is provided by the circadian
biorhythm. The rod discs are destroyed and engulfed at the day-time (when they
do not function), but the cone are destroyed and engulfed at nighttime when
they do not also function. It is depended on some factors. For example, at
daytime the rods are degenerated and engulfed because their discs contain much
vitamin A, which actively destroys the rod disc membranes. In addition the
cyclic adenosine monophosphate (cyclic AMP) inhibits the disc destroying but at
daytime the rod discs contain the cyclic AMP a few that leads to destroy them.
At night the rod discs contain much of the cyclic AMP protecting them from
destroying.
Accessory Ocular Organs
Eyelids, lacrimal
apparatus and external ocular muscles represent them.
The Eyelids
Their external surface is covered by the skin
with stratified squamous keratinized epithelium (Fig. 83). The skin includes
small hairs and sweat and sebaceous glands are associated. The internal surface
of eyelid is covered by the palpebral conjunctiva with the stratified squamous
non-keratinized epithelium. A stratified
squamous keratinized epithelium of skin is continuous with the stratified
squamous non-keratinized that of the internal surface of the eyelid. The
skeleton of the eyelid is formed by a mass of fibrous tissue called the tarsal
plate. Considerable thickness of eyelid is formed by fascicules of the
palpebral part of the orbicularis oculi muscle.
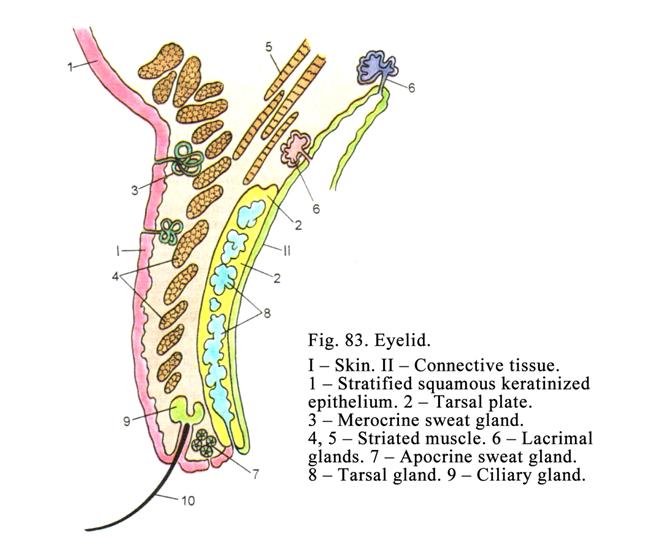
Along of the eyelid
margin the eyelashes are arranged. Some ducts of sebaceous glands open into a
hair follicle. Ducts of the modified sweat glands also open into the follicle.
On the deep surface of the tarsal plate sebaceous glands are located. Their
ducts open along of the eyelid margins. In the internal angle of the eye the
tertiary eyelid, covered by stratified squamous epithelium, containing the
mucous cells is lodged.
The lacrimal Apparatus
It consists of lacrimal glands,
lacrimal sack, and lacrimal duct opening into nasal cavity. The lacrimal glands
are compound branched tubuloalveolar glands. They release a secret consisting of
water (98%), chlorides (1.5%), albumins (0.5%), and mucus. The lacrimal fluid
contains lysozyme destroying bacteria.
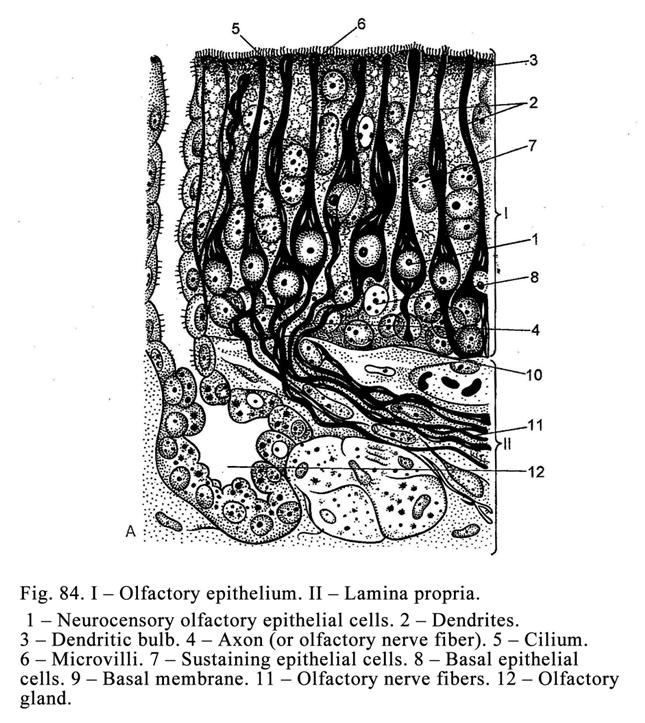
Lining of lacrimal sack
and lacrimal duct is a pseudostratified epithelium.
The Olfactory Organ Development and
Function
The organ (Fig. 84) is
lodged in olfactory area (olfactory epithelium) localized on the upper and
partly on the middle concha. The olfactory organ is derived from thickening
ectoderm near the cranial end of the neural tube. At first the olfactory buds
are formed. The buds migrate to the upper and middle conchae. There the
olfactory bud cells undergo differentiation to form olfactory and sustaining
epithelial cells. The olfactory cell bodies form axons and dendrites. The axons
are sent to the brain.
The Olfactory Mucosa Structure
The olfactory
epithelium, lying on the thick basal membrane, is pseudostratified, consisting
of: 1) neurosensory olfactory epithelial cells, 2) sustaining epithelial cells,
and 3) basal epithelial cells.
The neurosensory
olfactory epithelial cells are modified neurons. Each cell has a central part containing a round
nucleus. Two processes (distal and proximal) arise from the cell body. The
distal process (representing the dendrite) passes towards the surface of
the olfactory epithelium. It ends by the thickening (dendritic bulb) from which
a number of olfactory cilia arise. The cilia surface cell membrane bears
proteins perceiving smell. The receptive proteins receive the smell substances,
which are dissolved to make chemical reaction resulting in alteration of
permeability of the cilia cell membrane. The permeability of the cilia cell
membrane alteration provides nerve impulse.
The proximal process of each olfactory cell is axon. It passes into the subjacent connective tissue where it forms one fiber of the olfactory nerve reaching the olfactory bulb. The nerve fibers of the olfactory bulbs form the olfactory tract ending in a hippocampus and the hippocampal gyrus of the cerebral cortex, where the olfactory center is located. The cell body of the olfactory cell contains a rounded nucleus, mitochondria, Golgi complex, and rough ER.
The sustaining
epithelial cells
are column shaped. Their basal end lies on the basal membrane while their
apical (free) end reaches the olfactory epithelium surface. The cell nucleus
lies in the center of the cell, prominent organelles, microfilaments are also
present. The sustaining epithelial cells provide supporting function, release
secret (apocrine type of secretion), dissolve smell substances, and isolate
olfactory cells from each other.
The basal epithelial
cells are
triangular. They do not reach the luminal surface. They are the stem cells.
They divide to form neurosensory olfactory epithelial cells and supporting cells
to replace those that die, i.e. the olfactory epithelium is renewed in every 30
days.
Olfactory Glands
The olfactory
tubuloalveolar glands, surrounded by loose connective tissue, are located deep
to the basal membrane. The glands release fluid secretion dissolving smell
substances.
The Vomeronasal Organ
It is an additional
olfactory organ. It is made up of 2 tubules located to the lower part of the
nasal septum.
The Vomeronasal Organ Development
On the sixth week of the
uterine life the nasal septum epithelium lower part invaginates into the nasal
septum connective tissue as two tubules. On the seventh week the round cavity
of the vomeronasal tubules is formed. On the twenty-first week its sensory
epithelial cells and supporting epithelial cells are differentiated. From the
cell body of the neurosensory cells the peripheral process (dendrite) arises.
The dendrite end becomes thick to form a dendritic bulb. The second process of
the neurosensory cell (axon) joins with the same processes to form fascicles
passing into the brain.
The Vomeronasal Organ Structure
The vomeronasal organ
tubular distal ends are blind while their proximal ends open into nasal cavity.
The olfactory epithelial lining of the tubules contains 1) neurosensory
olfactory epithelial cells, 2) supporting epithelial cells, and 3) basal
epithelial cells.
The neurosensory
olfactory epithelial cells are columnar; they contain prominent organelles, and oval nucleus. From
their bodies peripheral processes are raised. The processes have thickened ends
or olfactory bulbs. The olfactory bulb surface bears a number of non-motile
olfactory cilia, having receptive proteins, which receive smells of secretion
released by sex glands of the opposite sex individual.
The central processes of
the neurosensory olfactory epithelial cells are joined with similar processes
to form nonmyelinated nerve fibers running to the additional olfactory bulb of
the brain.
The supporting
epithelial cells
are elongated. They contain oval nucleus, Golgi complex, ER, and mitochondria.
Their free surface bears microvilli. These cells release fluid secrete
dissolving smell substances.
The basal epithelial cells (stem cells) undergo division to form the new neurosensory olfactory
cells and supporting cells those that die.
The vomeronasal organ
influence the sex behavior of person and them emotional condition.
CHAPTER 12
THE EAR & GUSTATORY ORGAN
The Ear
It is divided into the
external ear, middle ear, and internal ear.
The External Ear
It consists of the auricle,
the external acoustic meatus, and the tympanic membrane.
The Auricle
It consists of a thin
plate of an elastic cartilage covered by true skin. In the skin there are hair
follicles, sebaceous and sweat glands.
The External Acoustic Meatus
The external acoustic
meatus wall consists of partly from elastic cartilage (in its outer part) and
partly from bone (in its inner part). A skin lines the meatus lumen. The skin
contains hairs, sebaceous glands, and ceruminous glands releasing the ear wax.
The Tympanic Membrane
It has an oval form and
is made up of three layers. The middle layer consists of fibrous tissue, which
is lined on the outside by skin and on the inside by mucous tunic, which is
continuous with the tympanic cavity.
The fibrous tissue of
the middle layer contains collagen fibers and some elastic fibers. The fibers
are arranged in two layers. In the outer layer they are placed radially while
in the internal layer they are arranged circularly. Between the fibers there
are fibroblasts. The mucous tunic is lined by simple squamous epithelium (but it may be cuboidal). To the tympanic
membrane mucous tunic the malleus is attached. From the malleus to the tympanic
membrane small arteries and nerves go out.
The Middle Ear
It includes the tympanic
cavity, the auditory tube, and ossicles (malleus, incus, and stapes)
The Tympanic Cavity
The tympanic cavity wall
is lined with the thin mucous membrane. The membrane is lined with simple
squamous epithelium (in same parts with cuboidal or columnar epithelium). The
lateral wall of the cavity is the tympanic membrane. On the tympanic cavity
medial wall there is oval window, closed by connective tissue ligament, to
which the stapes is attached, and round window closed by the secondary tympanic
membrane. The oval window separates the tympanic cavity from the scala
vestibuli while the round window separates it from the scala tympani of
cochlea.
The Auditory Tube
Owing to the auditory
tube the tympanic cavity communicates with nasopharynx. It is 1-
Ossicles
They are joined by
articulations of auditory ossicles, forming a chain that is attached on the one
side to the tympanic membrane, and at other end to the ligament closing the
oval window.
The Internal Ear
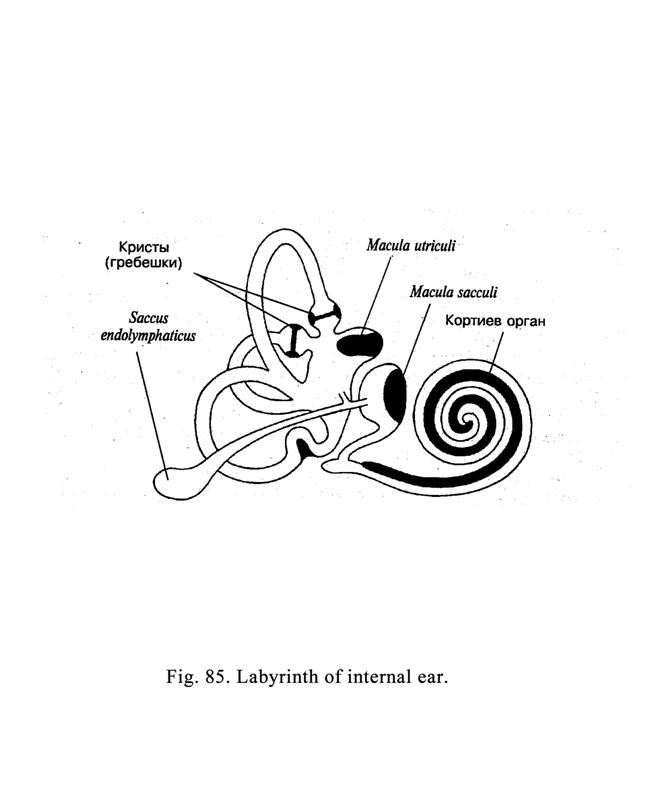
It consists of a bony
labyrinth and membranous labyrinth (Fig. 85). The membranous labyrinth lies
within the bony labyrinth. Both the bony labyrinth and membranous labyrinth are
divided into vestibular part and cochlear part. The cochlear part is the
hearing organ while the vestibular part is the equilibrium organ. In the bony
cochlea the cochlear membranous duct is located. In the vestibular part the
utricle and saccule, communicated with each other by utricle-saccularis duct,
are located. From the latter the endolymphatic duct (sac) arises. The duct
thickening end is applied to dura mater therefore if the internal ear is
inflammed, the dura mater is inflammed too. Within the bony semicircular canals
there are membranous semicircular ducts containing the circular kinetic balance
organs.
The Internal Ear Development
This is derived from the
thickened ectoderm locating near the forming brain. At first this thickening
invaginates into subjacent mesenchymal tissue and separates from ectoderm to form
the hearing vesicles (right and left). The vesicle lumen is lined with
pseudostratified epithelium and filled with fluid. The vesicle median wall
comes in contact with hearing nerve ganglion. In the process of the further
formation nerve ganglion and hearing vesicle are divided into vestibular and
cochlear parts. The cochlear part includes the future membranous cochlear duct
but the vestibular part includes the future membranous saccule, utricle, and
semicircular ducts.
The future membranous
duct grows and enters the developing bony canal, which forms cochlea. Between
the walls of the cochlear duct and bony canal two spaces are formed: scala
vestibuli and scala tympani containing the perilymph. In the process of growth
the bony canal is coiled up two and one second revolutions round the bony axis
(modiolus). The membranous cochlear duct also is coiled up.
At the same time the vestibular part
is formed to make the membranous saccule and utricle, and three semicircular
ducts dilated at the place of their attachment to the utricle. The dilatation
is called the membranous ampulla. On the outside of the membranous vestibular
labyrinth the bony labyrinth is formed.
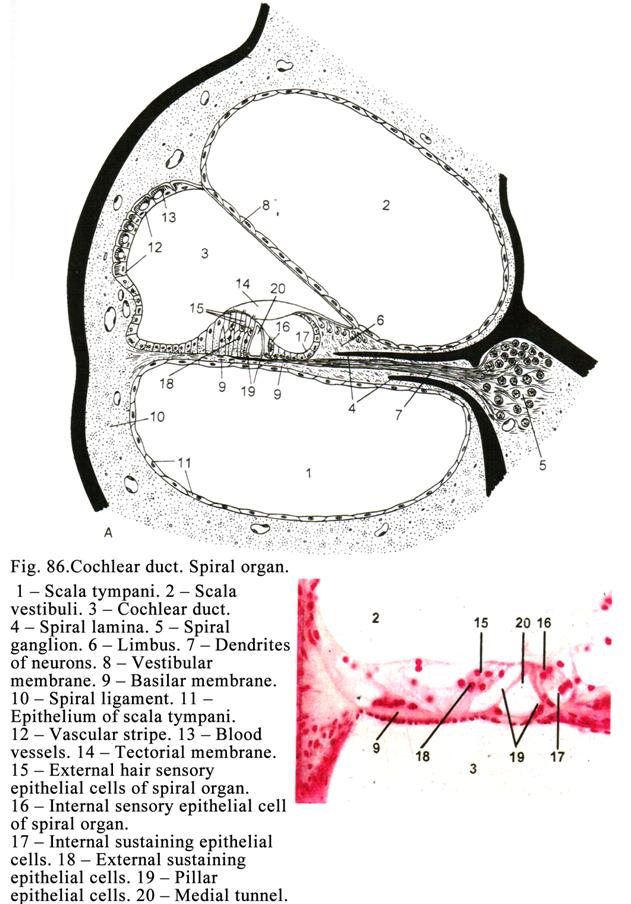
The Cochlear Labyrinth
The cochlear part of the
labyrinth is represented by bony cochlea, within which the cochlear membranous
duct is located (Fig. 86). The bony cochlea is coiled up two and one-second
revolutions round the bony axis called the modiolus. The length of the bony
cochlea is
The
Cochlear Duct (Membranous Labyrinth of the Cochlea)
The cochlear duct
located within the bony cochlea is triangular space. Its length is
The
Vestibular Membrane Structure
It is represented by the
fibrous connective tissue consisting of the collagen fibers, embedded into the
amorphous matrix. The vestibular membrane external surface is covered by an
endothelium while its internal one is covered by the simple squamous
epithelium. The vestibular membrane internal margin is attached to the spiral
crest and external one is attached to the bone cochlea periosteum.
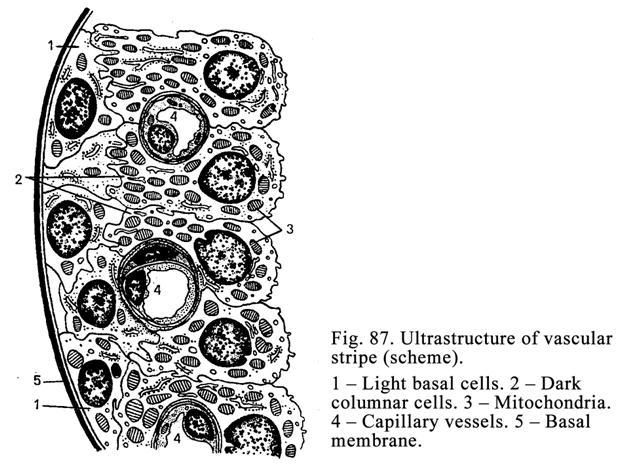
The External Wall Structure
The external wall is
represented by the bone cochlea periosteum, covered by the vascular stripe
(Fig. 87), containing the wide light cells and high dark cells. Blood capillary
enter the vascular stripe. The vascular stripe releases the liquor (endolymph)
filling the membranous cochlear duct.
The Spiral Membrane Structure
In is made up of
collagen fibers embedded into the amorphous matrix. Collagen fibers consist of
thin (30 nm in diameter) fibrils. The fibrils join each other by thinner
fibrils. Collagen fibers are similar to the strings. In the basal part of the
spiral membrane collagen fibers lens is about 105 mm (short strings) while in its
apical part it is about 505 mm (long strings). The short strings
perceive high sound but the long strings perceive low sound. An external
surface of the spiral membrane is covered by endothelium while on its internal
surface a membrane is located. The spiral organ is located on it. The external
margin of the spiral membrane is attached to the spiral ligament while its
internal margin is attached to the tympanic labium of the spiral crest. The
epithelium, covering the luminal surface of the cochlear duct (simple squamous
epithelium of the vestibular membrane, epithelial cells of the vascular stripe,
and epithelial cells of the spiral organ) is derived from the ectoderm.
The Spiral Organ (Organ of Corti)
It is placed on the
basal membrane. The spiral organ (Fig. 86) includes internal and external hair
sensory epithelial cells, internal and external sustaining epithelial cells,
and internal and external pillar epithelial cells (Fig. 88.) The internal and
external pillar epithelial cells are arranged in one line limiting the internal
tunnel filled by endolymph. The tunnel is the center of the spiral
organ. The cells
(pillar,
sustaining, and hair), located
between the modiolus and internal tunnel, are called the internal cells, but
those, localized between an external wall of the cochlear duct and the inner
tunnel, are called the external cells.
The Internal Sensory Epithelial
Sells
They are arranged in one
line. These cells are pear shaped (Fig.88A). Their amount is about 3500. Their
rounded (oval) basal end is placed on the apical part of the internal
sustaining (internal phalangeal) epithelial cells. The round nucleus of the
hair cells is located on their basal part. Their cytoplasm contains common
organelles and filaments. On the apical (free) surface of the hair cells the
cuticle plate is located, from which 60 immovable hairs (stereocilia) arise.
The length of the hairs is 2-5 mm. The cell membrane of the hairs bears choline
receptors.
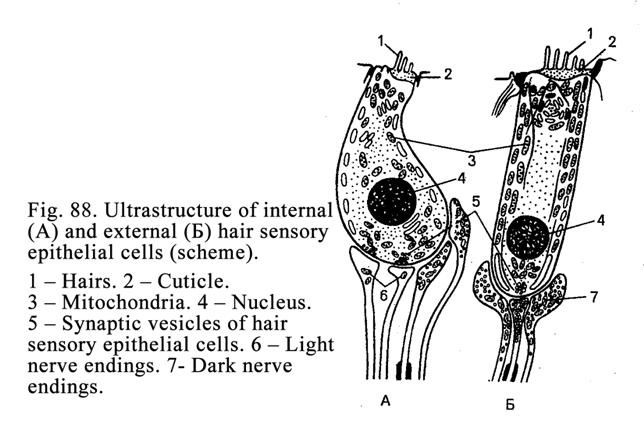
The External Hair Sensory Epithelial
Cells (Fig. 88Į)
They form 3-5 rows.
Their amount is 12000-20000. The cells have columnar shape. Their base lies on the
external sustaining (phalangeal) epithelial cells. The round nuclei of the hair
cells are located in the middle of the cell. The cells contain ribosomes, ER,
and mitochondria. On their free surface there is cuticle plate from which hairs
arise. The hairs are arranged as letter V (Fig. 89). The cell membrane of the
hairs bears choline receptor proteins and enzyme (acetyl choline esterase)
destroying the acetylcholine. The hairs contain actin and myosin filaments. Due
to filaments these hairs can erect after their contact with the tectorial
membrane.
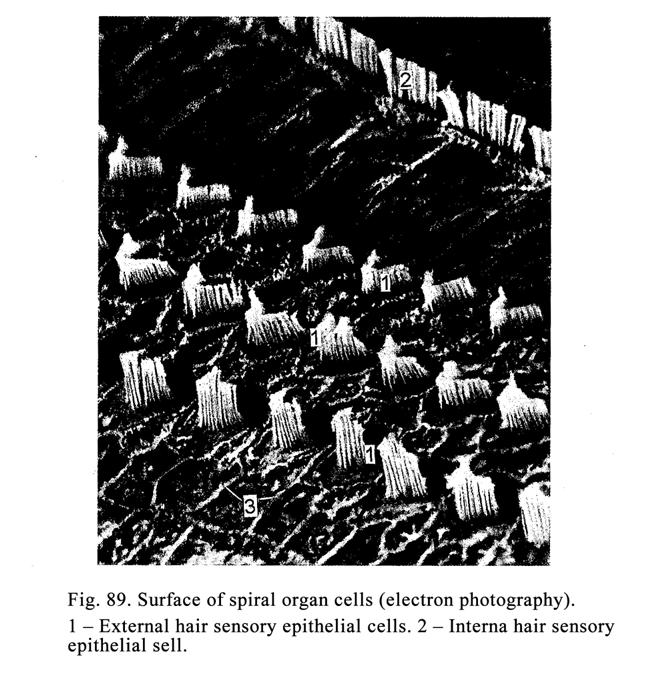
Internal Sustaining (Phalangeal)
Epithelial Cells
They have columnar
shape. Their base lies on the basal membrane. On the cell apical part there is
depression, where the internal hair cell bases are located. The cells contain
common organelles, abundant filaments, and round nucleus located in the center
of the cell. From the apical surface of the internal phalangeal cells the
phalangeal process arises separating the internal hair cells from each other.
External Sustaining Epithelial Cells
They are divided into
external (phalangeal) cells, external limiting sustaining epithelial cells, and
external sustaining epithelial cells.
The external
phalangeal epithelial cells are column shaped. Their basal end is placed on the basal membrane
while apical part has depression, where the base of the external hair cells is
located. The round nucleus of the cell is placed in the center of cell.
Cytoplasm contains common organelles and microfilaments. From the apical part of
the cells phalangeal process arises, separating external hair cells from one
onother.
The external limiting
epithelial cells
are column shaped. The basal part of every cell is placed on the basal
membrane. They are shorter than phalangeal cells. The free surface of the cell
bears microvilli. Their cytoplasm contains common organelles, filaments, and
glycogen inclusions as well. The round nucleus is locates in the center of the
cell. The cell is believed to provide the nutrient function.
The external sustaining
epithelial cells
are cuboidal. They join the epithelium of the vascular stripe.
External and Internal Pillar
Epithelial Cells
They limit the internal
tunnel. Their wide base is placed on the basal membrane. The round nucleus is
placed in the basal part of the cell. The apical ends of the internal and
external pillar cells join each other to form internal triangular tunnel filled
with endolymph.
The Tectorial Membrane
The tectorial membrane
is represented by the connective tissue lamina consisting of collagen fibers
embedded into gelatinous matrix. The membrane internal margin is attached to
the vestibular labium of the spiral crest. Its external margin is free and
floats in the liquor (endolymph) above the spiral organ. The tectorial membrane
length is similar to that of the spiral organ (
Some Elementary Facts about the
Mechanism of Hearing
Sound waves, entering
the external acoustic meatus reach the tympanic membrane providing its
oscillations. The latter are transmitted through the chain of ossicles to
perilymph of the scala vestibular of cochlea. The oscillations (waves) travel
to the apex of the cochlea. At this point (called the helicotrema) the scala
vestibule joins the scala tympani. The sound waves pass from scala vestibule
into the scala tympani through the helicotrema and again travel the whole
length of the cochlea to its basal end. On this way vibrations (oscillations)
are set up in the perilymph and through it in the basilar (spiral) membrane.
Movements of the basilar membrane produce forces that result in friction
between the hairs of hair cells and the tectorial membrane. These friction
leads to bending of the hairs. In addition the hair choline receptors of the
hair cell membrane receive the acetylcholine presenting in the cochlear duct.
It leads to the alteration of the cell membrane of the hair cell permeability.
The alteration generates the nerve impulse formation. After it the enzyme
destroys acetyl choline received by receptors.
Nervous impulse is
transmitted to the dendrite of the bipolar neuron of the spiral ganglion. The
neuron axon transmits the impulse to the anterior cochlear nucleus or the
posterior cochlear nucleus. If the impulse is transmitted to the neuron of the
anterior nucleus, its axon transmits it (impulse) to the superior olivary
nucleus or trapezium nucleus. Here the third neuron of hearing tract is
present. The third neuron axon travels to the opposite side of the pons to form
lateral lemniscus. The neuron axons (nervous fibers) of the lemniscus travel to
the inferior colliculus and the medial geniculate body (here the fourth neuron
is located). The fourth neuron axon travels to the temporal lobe of the
cerebral cortex where the central end of the cochlear (hearing) tract is
located.
If the bipolar neuron
axon transmits the impulse to the posterior cochlear nucleus (to the second
neuron of the cochlear tract), the axon of this neuron transmits the impulse to
the lemniscus lateral nucleus neuron. The axon of this neuron conducts the
impulse to the medial geniculate body or the inferior colliculus. The axons of
these neurons transmit the impulse to the neuron of the temporal gyrus of the
cerebral cortex.
The Vestibular Membranous Labyrinth
It is represented by a
saccule and utricle, and by semicircular ducts (anterior, lateral, and
posterior). The place, where the duct is attached the utricle, is a dilatation
called the ampulla. Within the utricle and saccule there are maculae. Within
the ampulla there is the ampullary crest. The saccule and utricle are
communicated by the endolymphatic duct. From the duct the endolymphatic sac
arises, ending by the dilatation, which contacts the dura mater. Therefore
inflammation can be transmitted from the internal ear to the dura mater.
Maculae of the Utricle and Saccule
(Fig. 90)
The lumen surface of the
utricle and saccule is lined by a simple squamous epithelium. At the macula place
the epithelial cells become cuboidal or columnar. The cells of the macula are
located on the basal membrane. The macula includes sustaining epithelial cells
and hair sensory cells. On the cells of the macula thick membrane is located.
It is called the statoconium membrane, consisting of the jelly-like substance,
including statoconiums or otoliths, containing calcium carbonate.
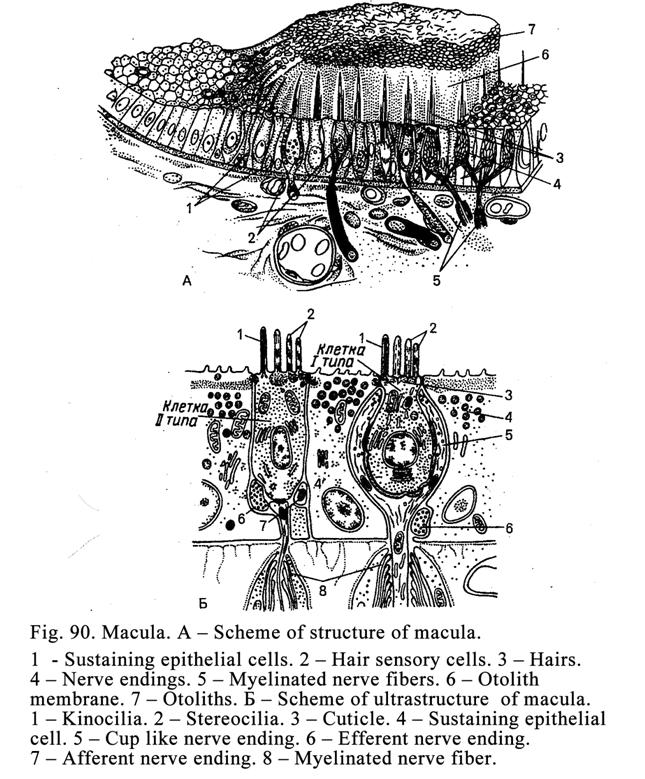
From the apical end of
the sensory cells hairs arise (about 80). Their length is about 40 mm. One hair is movable (kinocilium).
The other hairs are immovable (stereocilia). The kinocilium is located on the
hair cell apical margin (opposite the stereocilia). The hairs of the sensory
epithelial cells enter the statoconium membrane. The hairs cells are divided
into 2 types: 1) piriform epithelial cells (type I) and 2) columnar epithelial
cells (type II).
The type I cells are pear shaped. Their basal end
contains round nucleus. Cytoplasm contains mitochondria, rough ER, and
ribosomes. The cell basal end is surrounded by a number of nervous fiber
terminals forming the calyx.
The type II cells are columnar. A few nervous fibers
contact with cell basal end to form spray nerve endings. The cell internal
structure is similar to that of I type.
The sustaining
epithelial cells
are placed on the basal membrane. They provide supporting and nutrient
functions.
The maculae of the
utricle and saccule are concerned with 1) perceive the straight movement
alteration, 2) change in position of the head, and 3) vibration.
Mechanism Perceiving Straight
Movement Change and Head Position Change
The statoconium membrane takes part
in the perception of the movement and
head position. When the movement stops, the statoconium membrane moves in 1-2 mm and makes the hairs of the sensory
epithelial cells to bend. If the stereocilia bend to the kinocilium, the
sensory epithelial cell becomes are excited.
If the stereocilia bend from the kinocilium, the sensory epithelial cell
is inhibited.
The hair sensory epithelial cells of
the macula (or utricle) are arranged so that if the statoconium membrane moves
in any direction some cells are excited while the others are inhibited.
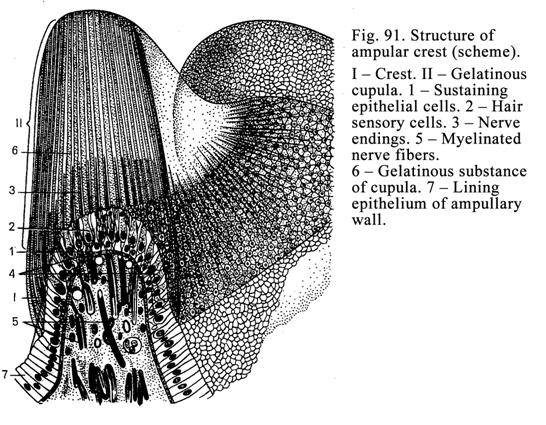
The change in position of the head
is perceived similar to the straight movement. If the head or trunk have
horizontal posicion, the statoconium membrane moves down in 1-2 mm to bend the sensitive cell hairs
as a result some cells provide excitant other cells provide inhibiting
impulses.
Ampullary Crests
These crests are present
in the ampulla of the semicircular ducts. The lining of the semicircular duct
ampulla is simple squamous epithelium. At the ampulla crest the epithelial
cells become columnar. Each crest (Fig. 91) is an elongated ridge projecting
into the ampulla, and reaching almost up to the opposite wall of the ampulla. The
ampulla sensory epithelial cells are divided into 2 types: type I (pear-form
cells) and type II (columnar cells). The hairs of the sensory epithelial cells
extend into the gelatinous (protein polysaccharide) substance, covering the
crest and called the cupula. The latter length is about
The ampullary crests
perceive the angular movement alteration. For example, if the angular movement
stops, but the cupula bends forwards therefore the sensitive cell hairs bend to
produce the impulses running to the skeletal and extraocular muscles.
Traveling Nerve Impulses from
Vestibular Labyrinth
From the sensory hair
epithelial cells of the vestibular labyrinth the impulses are transmitted to
the neuron in vestibular ganglion (the first neuron). From this neuron nervous
fiber travels to the cerebellum or to the vestibulocochlear nucleus (the second
neuron is located there). This neuron axon runs to the cerebral cortex, where
the vestibular center is placed. At the same time some neuron axons travel to
the spinal cord, reticular formation, and cerebellum.
Innervation of the Vestibulocochlear
Labyrinth Structures
Apart from the afferent
nerve fibers the spiral organ, maculae and ampullary crests receive nervous
impulses from efferent nervous fibres traveling from an inferior olive. The
afferent and efferent nerve fibers form nervous plexuses near the external hair
epithelial cells of the spiral organ (external spiral plexus) and in the
internal ones (internal spiral plexus) To the external hair cell efferent nerve
fibers predominantly travel while to the internal hair cells the afferent nerve
fibers predominantly run.
The Internal Ear Blood Supply
The branch of the upper
cerebral artery is divided into vestibular and cochlear arteries. The
vestibular artery supplies the vestibular apparatus (the maculae of the saccule
and utricle, semicircular ducts, and ampullary crests) while the cochlear
artery supplies the cochlear apparatus (the spiral ganglion, internal part of a
spiral membrane, and vascular stripe).
The Outflows of Venous Blood from
the Internal Ear
It is provided by the
venous plexuses of the cochlea, utricle, saccule, and semicircular ducts.
Age Dependent Changes of the Ear
In the ear of the old person
the ossification in the region, where the stapes is attached to the ligament of
the oval window, may occur. At the same time some hair cells, perceiving
hearing sound waves and converted them into nervous impulses, may die. It
results in the low of hearing. The low hearing produced by the ossification of
the oval window ligament can be corrected with the hearing aid. The death of
the hearing pathway neurons or hair sells, perceiving the sound, cannot be
corrected by the hearing aid.
Gustatory
Organ
The gustatory organ
(Fig. 92) is represented by gustatory buds (taste buds) located in stratified
squamous epithelium, which cover the fungiform papillae, papilla vallate, and
foliate papillae of the tongue. The taste buds can be located in the epithelium
of the lips, palatine arc, and epiglottis. In comman the gustatory organ
constiutes about 2000 buds.
The taste buds are
derived from the tongue papilla epithelium through embryonic life. Terminals of
the facial nerve, glossopharyngeal nerve, and vagal nerve come up to the tongue
papilla epithelium. These terminals stimulate the differentiation of the
epithelial cells resulting in conversion of the latter into gustatory,
sustaining, and basal epithelial cells to form taste buds.
The Gustatory Bud Structure
The bud has ellipsoid shape. The
gustatory bud extends through the entire thickness of the epithelium. Each bud
has the small cavity opened to the surface through gustatory pore. The cavity
is filled by substance rich in phosphates, receptive proteins, and
polysaccharides. This mass is an adsorbent abling to adsorb gustatory products.
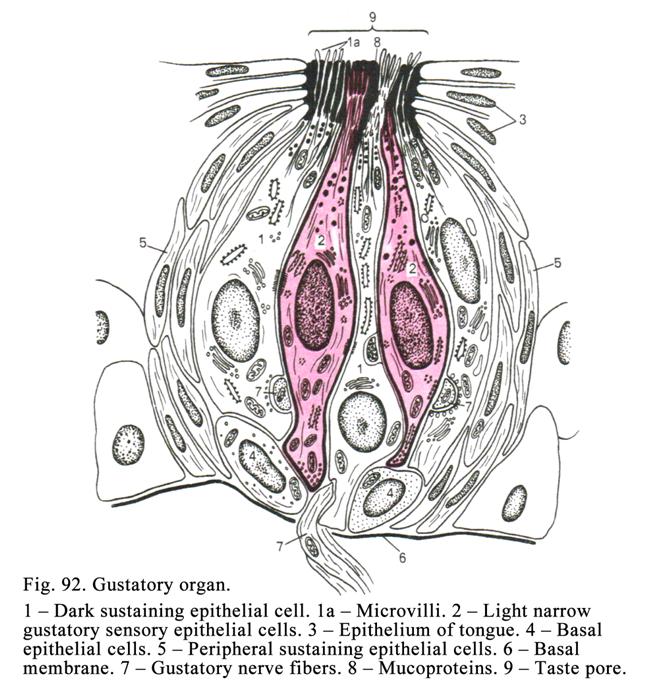
The gustatory bud is
made up of 50 cells including 5 cell types: 1) light narrow gustatory
epithelial cells, 2) light columnar gustatory epithelial cells, 3) dark
sustaining epithelial cells, 4) basal epithelial cells, and 5) sheath cells.
The first and second
gustatory epithelial cells are elongated. Their basal end is located on the
basement membrane. The membrane separates the bud from the subjacent connective
tissue. The free (apical) surface of the gustatory cells bears
microvilli having receptive proteins. Receptive proteins, perceiving the
gustatory substances, at the tongue tip perceive sweet substances while the
receptive proteins of the tongue posterior part perceive bitter taste. The
gustatory sensory epithelial cells contain oval nuclei, mitochondria and smooth
ER. The nervous terminals come up to the sensory cells to form synapses.
The sustaining
epithelial cells
are columnar in shape. They contain oval nuclei, located in the cell center,
Golgi complex, mitochondria, and rough and smooth ER. The cell basal end is
located on the basal membrane.
The sustaining
epithelial cells isolate the sensory cells from onother and release the
secretion, containing glycoproteins.
The basal cells are short and triangular in shape.
Their wide basal end is located on the basal membrane. The cells are able to
multiply by mitosis. They provide regenerative function, i.e., they produce
sustaining and sensory cells to replace those that are worn out (in 10
days).
The sheath cells are located in the peripheral part
of the taste bud and are C-shaped. They probably separate the taste bud from
the surrounding epithelial tissue, i.e., they provide the formation of the sheath
around the taste bud.
Formation and Movement of the
Gustatory Impulse
The receptive proteins
perceive taste substances. It leads to high permeability of microvilli cell
membrane resulting in the impulse production.
Through the synapse the impulse passes to the neuron, located in the
ganglion of VII, IX, and X (facial, glossopharyngeal, and vagal) cranial nerves
(the first neuron is there). The first neuron axon carries the impulse to the
brain stem nucleus (the second neuron is there). From the second neuron the
impulse travels to the salivary glands, tongue muscles, facial muscles, and
thalamus. In the thalamus the third neuron is located. The third neuron axon
runs to the fourth neuron located in the post central gyrus (cortical gustatory
center).
CHAPTER 13
CARDIOVASCULAR SYSTEM
This system is composed
of the heart, blood vessels and lymphatic vessels.
Blood Vessels
Blood vessels are
derived from mesenchymal cells of the yolk sac wall. On the third week of
uterine life mesenchymal cells unite to form blood islands. The central cells
of the islands convert into the blood cells while the peripheral cells convert
into the endothelial cells of blood vessels. Thereafter within the fetus body
blood cells and blood vessels are also formed from the mesenchymal islands.
Then the fetus body vessels join those of the yolk sac and chorion villi.
The body blood vessels
constitute communicating system of tubes providing transport, nutrition, and
metabolic functions. In addition the system regulates blood circulation in
organs and tissues.
Classification of Blood Vessels
Blood vessels are
classified into arteries, veins, and microscopic vessels (capillary vessels,
arterioles, arteriovenous anastomosis, and glomera). The arteries take the blood
from the heart. The veins return the blood to the heart. Arteries carry
oxygenated blood except the umbilical and pulmonary arteries, which carry
deoxygenated blood. Veins carry deoxygenated blood except the pulmonary and
umbilical veins, which carry oxygenated blood.
The artery wall is
composed of 3 tunics: 1) internal, 2) middle, and 3) external. (The vein wall
is also composed of the 3 same tunics).
The arteries are
classified into 3 types: 1) elastic artery (aorta and pulmonary artery), 2)
hybrid artery (carotid and subclavian arteries), and 3) muscle artery (the rest
arteries).
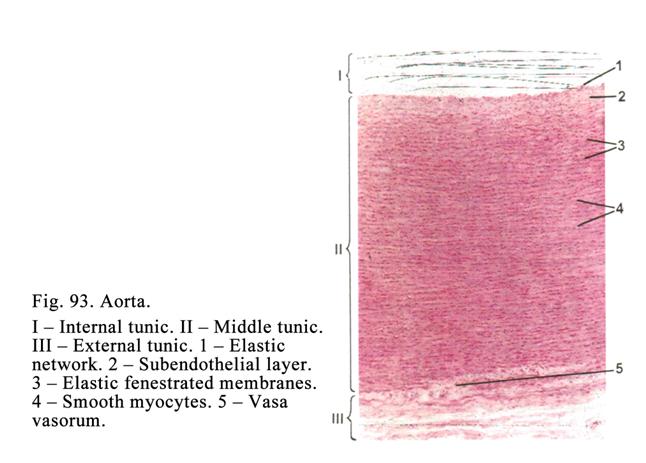
The Elastic Artery Type
The elastic artery type
wall (Fig. 93) is composed of three tunics: internal, middle, and external.
The Internal Tunic
The internal tunic of the aorta is composed of 3
layers: endothelial layer, subendothelial layer, and the plexus of elastic
fibers.
The endothelial layer is represented by flat polygonal
endothelial cells containing one or some oval nuclei. Their cytoplasm is poor
in common organelles except mitochondria. The cell membrane invaginates into
cytoplasm of the cell to form caveolae. The cell cytoplasm contains pinocytotic
vesicles. The luminal surface of the endothelial cells bears microvilli
enlarging the cell surface. The length of the endothelial cells is about 500 mm their width is about 140 mm.
The subendothelial
layer thickness is
about 15% from the aorta wall thickness. It is represented by thin connective
tissue including thin collagen and elastic fibers, fibrocytes, star-like stem
cells, smooth muscle cells, and fundamental substance. The latter contains
sulphate glycosaminoglycans (in elderly cholesterol and fat acids appear).
Interlacing elastic
fibers arranging circularly and longitudinally represents the plexus of elastic
fibers.
The Middle Tunic
The aorta middle
tunic consists of 50-70 elastic fenestrated membranes. Between the
membranes there are smooth muscle cells, thin collagen fibers, and elastic
fibers.
The External Tunic
It is made up of
connective tissue consisting of collagen fibers, macrophages, mast cells, and
adipose cells. In the external tunic there are also blood vessels (vessel of
vessel or vas vasorum), and nerves.
The aorta provides 1)
transport of blood 2) pushing the blood distally. Due to its elasticity it is
dilated in systole and in diastole its wall comes back to its original size
pushing the blood distally into the smaller arteries.
The Dynamic Conditions within the
Aorta
The systolic pressure
within the aorta is about
Hybrid Artery Types
They are presented by
subclavian and carotid arteries. Their internal tunic is composed of 3
layers: 1) endothelial lining, 2) subendothelial layer, and 3) internal elastic
membrane (this membrane is absent in elastic artery type).
The Middle Tunic
It is composed of about
25% of elastic fenestrated membranes, 25% elastic fibers, and 50% of smooth
muscle cells.
The External Tunic
It is made up of a
connective tissue including vas vasorum and nerves. In the internal layer of
the external tunic there are longitudinal fascicles of the smooth muscle cells.
The Muscular Artery Type
This type includes
arteries, having middle and small caliber, localized in the human body and its
organs.
The internal tunic of the arteries is made up of 3
layers: 1) endothelial lining, 2) subendothelial layer, and 3) very prominent
elastic membrane (Fig. 94).
The middle tunic is predominantly composed of smooth
muscle cells arranged circularly. Among the muscle cells there are collagen
fibers and elastic fibers interlacing with the internal elastic membrane and
passing into the external tunic to form the elastic framework. Due to the
framework the artery cannot be collapsed.
Between the middle and
external tunic there is the external elastic membrane, less prominent than the
internal elastic membrane.
The external tunic consists of a loose connective
tissue.
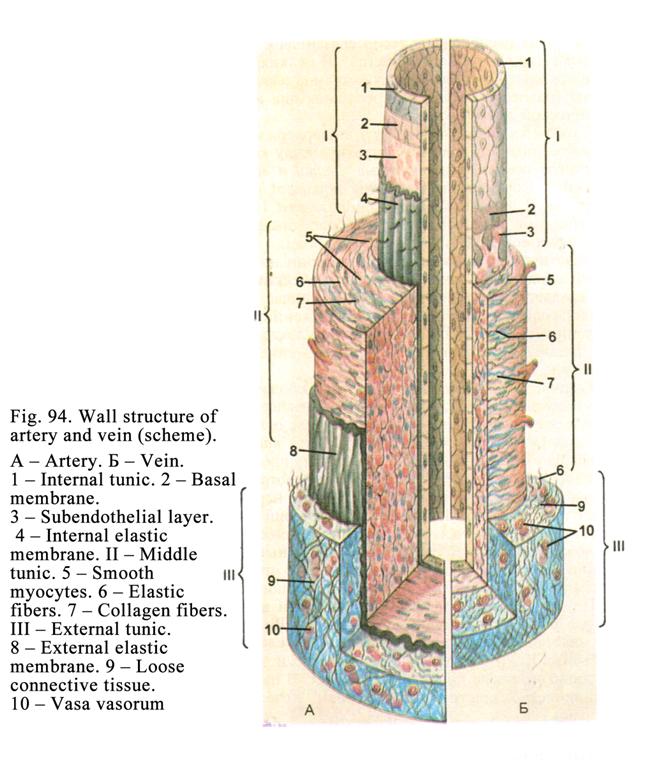
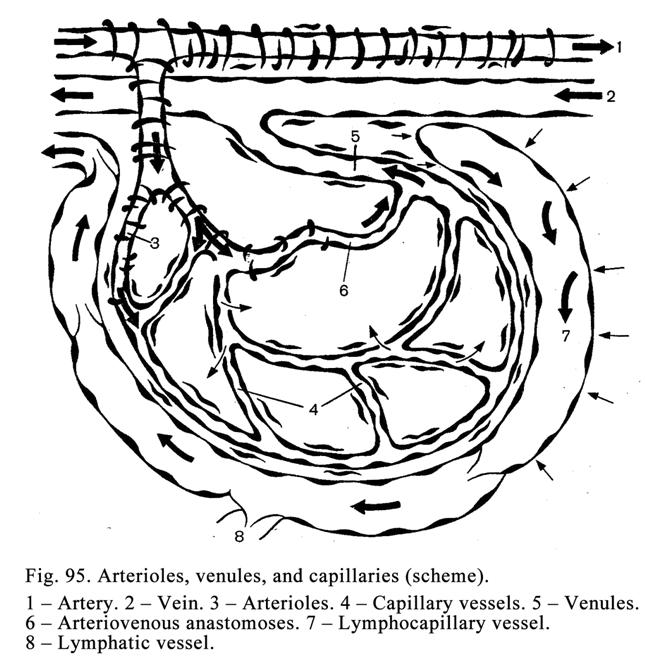
Microcirculstory bed
Microvessels
are arterioles, capillary vessels, venules, arteriovenous anastomoses, and
lymph capillary (Fig. 95). Micro vessels provide 1) metabolism, 2) blood flow regulation, 3)
blood accumulation, and 4) drainage of the connective tissue fluid.
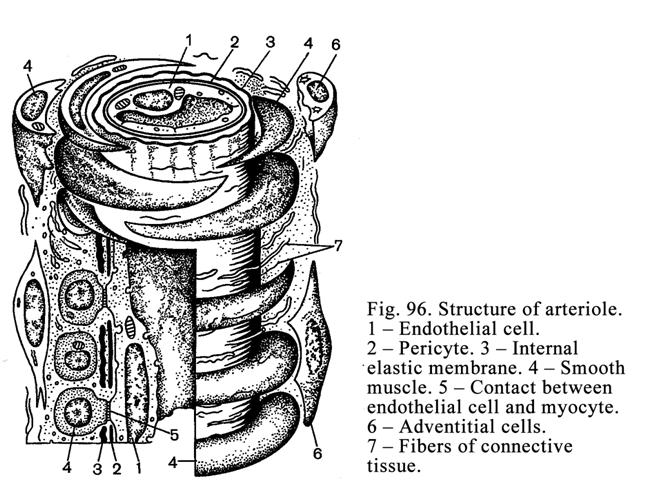
The arteriole wall (Fig. 96) structure
is similar to that of the artery. The endothelium, subendothelial layer, and
internal elastic membrane (having pores) represent its internal tunic.
Through pores of endothelial cells contact with the smooth muscle cells of the
middle tunic is formed. Their contacts allow adrenalin transmission from the
blood to the smooth muscle cells to produce their contraction. Here nervous
endings also regulate contraction/relaxation of the smooth muscle of the
arterioles. All layers of the internal tunic of the arterioles are very thin.
1-2 rows circularly arranged smooth muscle cells compose the middle tunic of
the arteriole.
The external tunic of the arteriole wall consists of
the thin layer of the connective tissue.
Classification of the Arterioles
The arterioles are
divided into muscular arterioles (100-50 mm in diameter) and terminal arterioles (less
than 50 mm in diameter). In the places, where
the terminal arterioles arise from muscular arterioles, and precapillary
vessels arise from the terminal arterioles, there are sphincters, consisting of
circularly arranged smooth muscle cells, regulating the blood flow in the
vessels.
The arterioles provide
1) regulation of the blood flow in organs and tissues and 2) the blood pressure
regulation.
Capillary Vessels
The capillary vessel
diameter varies from organ to organ. The smallest capillary vessels (4-7 mm in diameter) are in the skeletal
muscles, lungs, and nerves. Larger ones (8-11 mm) are in the skin and mucous membranes. If the
capillary vessels are about 30 mm in diameter, they are called the sinusoids.
The sinusoids are located in the hemopoietic and lymphatic organs, endocrine
glands, and liver. The largest capillary vessels or lacunae (more than 30 mm) are in the rectal column zone and
the corpus spongiosum and corpora cavernosa of the penis.
Capillary vessels form
the capillary plexus (capillary network). In addition they can form capillary
arches or loops in the intestine villi, skin papillae, and capsule joint villi.
The capillary vessel end, arising from the arteriole, is called the arterial
capillary vessel (end). The capillary end, entering the venule, is called the
venous capillary vessel (end).
The venous capillary end
is wider than arterial one in 2-2.5 times. The venous capillary end endothelial
cells contain more number of mitochondria and microvilli.
The capillary vessels
can form glomera (in the kidney). Capillary vessels can arise from the
arteriole and enter ones (kidney). They can arise from venules and enter ones
(hypophysis cerebry portal system).
The arrangement of the
capillary network and its density varies from tissue to tissue. The density is
the greatest in tissues having high metabolic activity. For example, in
skeletal muscle per1 mm2 there are 2000 sections of the capillary vessels
while in skin there are only 40.
In every tissue about
50% of capillaries do not function. In common exertion their walls are
collapsed. If functional exertion of the organ is increased these capillaries
become active.
The Capillary Vessel Wall Structure
(Fig. 97)
The capillary wall is
composed of 1) endothelium, 2) layer of pericytes, and 3) external layer,
consisting of fine fibers and cells.
The endothelial layer is composed of flat polygonal
endothelial cells of various size (5 to 75 mm in length). The luminal surface of the
endothelial cells, covering by glycocalyx, bears microvilli increasing their
surface. The endothelial cell membrane forms a great amount of caveolae. The
cell cytoplasm contains many pinocytotic vesicles. It is evidence that the
metabolism of the cell is high. At the same time the endothelial cells contain
a small amount of common organelles. In some capillary vessels there are
fenestrated endothelial cells i.e. the endothelial cells have pores. They are
called the fenestrated capillary vessels. Such capillaries are located in the
hypophysis cerebry and glomera of kidneys. The cytoplasm of endothelial cells
contains an alkaline phosphatase and other enzymes. Endothelial cells of the
venous end of capillaries make folds similar to the valves regulating the blood
flow in veins.
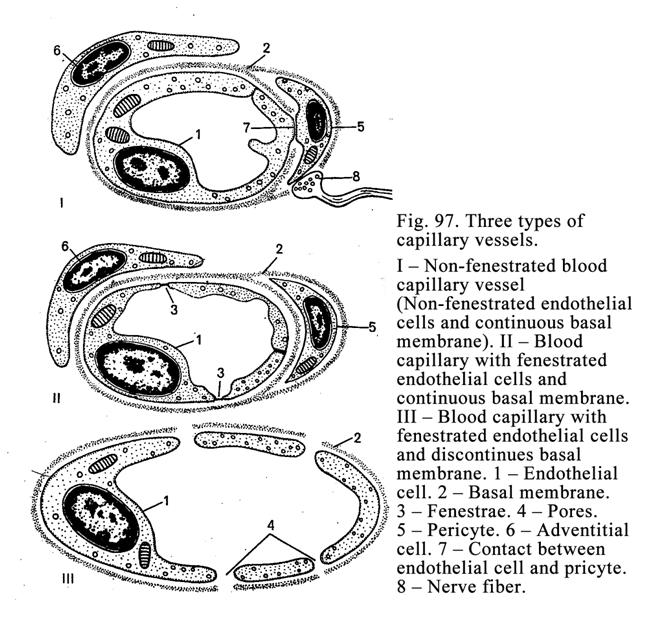
Endothelial cells
provide many functions. 1. They prevent the blood clotting. 2. They take part
in the capillary vessel basal membrane formation. 3. They take part in the blood
flow regulation (they release factors stimulating/inhibiting contraction of the
smooth muscle cells of capillaries).
4. They provide
barrier unction. 5.
They release factors stimulating the new blood vessel formation. 6. They
release some enzymes.
The capillaries basal
membrane is thick.
It contains adenosine three-phosphatase. In some capillaries the basal membrane
has pores or slits. The basal membrane provides selective and barrier
functions.
The pericytes are localized in the slit of basal membrane.
They have branches, including filaments, resembling myofilaments of the smooth
muscle cells. The pericytes branches round the capillary wall. These cells
contact with endothelial cells through pores in the capillary basal membrane.
The pericytes provide: 1) contraction due to the filaments, 2) owing to the
microfilaments the supporting cytoskeleton formation, 3) participation in
regeneration of the blood vessel wall due to the myofibroblasts, which are able
to convert into the smooth muscle cells of blood vessel wall, 4) stimulation of
the endothelial cell mitosis owing to the contact between the pericytes and
endothelial cells, and 5) participation in the capillary vessel wall membrane
formation due to the rough ER present in the cell.
The external layer
consists of fine collagen and elastic fibers, connective tissue cells, and its
fundamental substance.
Classification of Capillaries Depend
on their Wall Structure
There are 3 types (Fig.
97) of capillaries: 1) capillary wall, consisting of non-fenestrated
endothelial cells, and the continuous basal membrane (type I), 2) capillary
vessel wall, consisting of fenestrated endothelial cells, and the continuous
basal membrane (type II), and 3) capillary wall, consisting of fenestrated
endothelial cells, and discontinuous basal membrane (type III).
The first type is located in the skeletal muscles,
lungs, nerves, and mucosal membrane. The second type is located in the
glomera of kidney and intestine villi. The third type (sinusoid
capillaries) is located in the liver and hemopoietic and lymphatic organs. The
wall of the sinusoid capillary vessels is great permeability. It facilitates
the mature blood cells to pass from the hemopoietic organs into the sinusoid
capillaries.
The major function of
capillaries is metabolic one. Four factors provide this function due to: 1) the
thin wall of the capillaries, 2) slow blood flow (0.5 mm/sec.), 3) extensive
external surface of all capillaries with surrounding tissues (
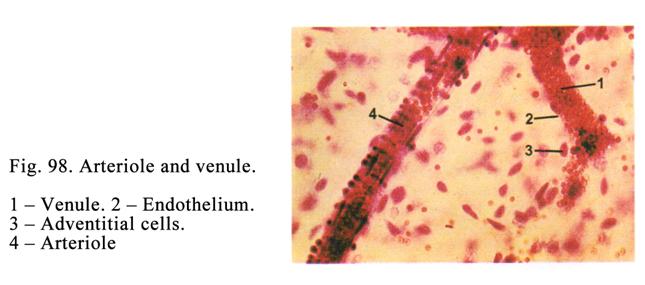
Venules
There are 3 types of
venules: 1) postcapillary venules
(they are 8-30 mm
in diameter), 2) collecting venules (30-50 mm), and 3) muscular venules (50-100 mm).
The wall of the
postcapillary venules is similar to that of the capillaries. They are
different only in amount of pericytes i.e., postcapillary venule wall contains
more pericytes but there are no myocytes there.
The wall of the
collecting venules includes endothelial cells, some smooth muscle cells in
its middle layer and thicker external layer, than that of postcapillary
venules.
The wall of the
muscular venules is made up of 1-2 layers of smooth muscle cells in its
middle layer.
Through the wall of the venules
metabolic substances from the surrounding tissues pass into the venules and
blood cells pass from the venules into the surrounding tissues.
Arteriovenous Anastomoses
These are the vessels (Fig.99)
through which the blood flows direct from the artery into the vein while the
blood does not flow into capillaries. When the anastomoses are closed, the
blood flows into the capillaries. The length of the anastomoses may be
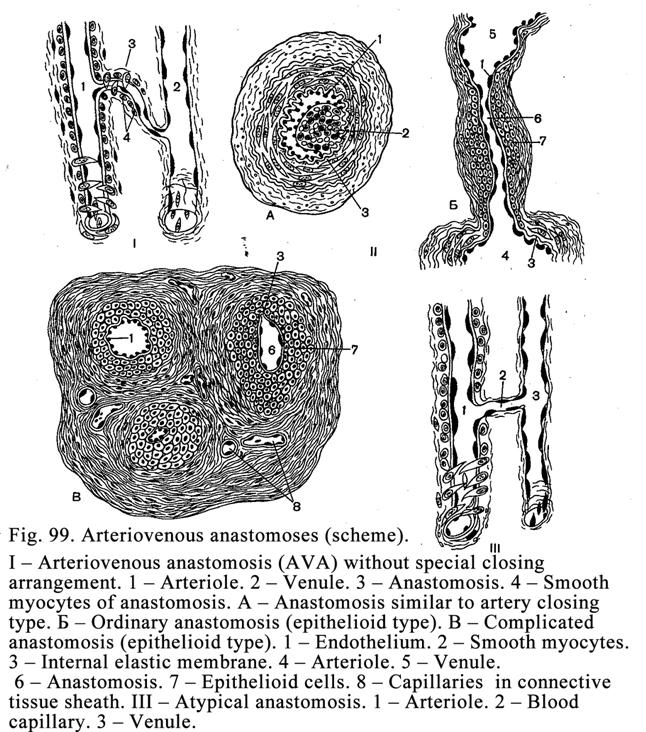
Atypical Anastomoses
The arterioles and venules are connected
by capillary type vessels (Fig. 99 III), but having a large size. These
channels run between arterioles and venules. Isolated smooth muscle cells can
be found in their wall. The blood, flowing through these vessels, is mixed as
oxygen passes into the surrounding tissues from the vessel while carbon dioxide
passes into the blood vessel from the surrounding connective tissue.
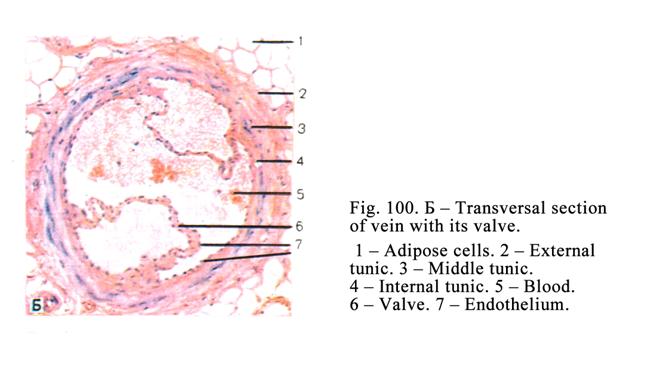
Arteriovenous
anastomoses 1) regulate blood flow, 2) add oxygenated blood into deoxygenated
blood, 3) carry the blood from arterioles into venules when the capillary
vessels are compressed by a pathological process, and 4) increase pressure in
the venules.
Veins
Their wall (Fig. 100) is
composed of 3 tunics: internal, middle, and external.
The veins are classified
into 1) muscular vein types and 2) trabecula vein types. The muscular vein
types are divided into 1) veins containing scanty smooth muscle cells in their
wall, 2) veins containing moderate amount of the smooth muscle cells, and 3)
veins containing great amount of the smooth muscle cells. In the upper part of
the human body there are veins containing a few smooth muscle cells. In its
middle part there are veins containing moderate amount of them. In the human
body lower part there are veins containing a lot of smooth muscle cells.
Valves of Veins
The most of veins
contain valves (Fig. 100. 6), which allow the flow of blood towards the heart.
The cusps of the valves are folds of an endothelium, where connective tissue
fibers are located. There are no valves in very small veins, in the cranial
veins, in the vertebral veins, in iliac veins, vena cava, and some other veins.
Trabecula Vein Type
They are located in the
meninges, in the brain, in the retina, in bones, and in the spleen. As the
veins of the meninges, the brain and retina are located in the cranial part of
the body the blood flows to the heart without any contraction of the veins. The
vein walls of placenta, bones, and spleen are fused with surrounding tissue and
cannot collapse and delay the blood flow.
Veins Containing Scanty Smooth
Muscle Cells in their Walls
They are the small and middle size veins of the face, neck, upper part of the body, and the large size vein (superior vena cava). In the very thin subendothelial layer of their walls there are no smooth muscle cells. In the middle tunic some circular smooth muscle cells are located. Between the smooth muscle cells there is considerable layer of loose connective tissue containing collagen and elastic fibers.
The external tunic
consists of connective tissue containing a few longitudinal bundles of the
smooth muscle cells.
In the subendothelial
layer of the superior vena cava there are a few longitudinal smooth muscle
cells. The middle tunic of this vein is represented by a few circular bundles
of the smooth muscle cells and loose connective tissue. The external tunic of
the superior vena cava is made up of connective tissue containing the
longitudinal bundles of the smooth muscle cells. The vein external tunic is
thicker in 5-6 times than both internal and middle ones.
Veins Containing Moderate Amount of
the Smooth Muscle Cells
The typical one is the
ulnar vein. Its internal tunic is composed of 3 layers: endothelium,
subendothelial layer, and layer formed by interlacing elastic fibers (the
elastic fiber layer). The internal tunic of this vein provides formation of 12
valves, which promote to carry the blood towards the heart. In the middle tunic
there are circular smooth muscle fibers, collagen and elastic fibers. The
elastic fibers interlace with the elastic fiber layer and external tunic to
form the elastic framework of the ulnar vein. The external tunic of the ulnar
vein is represented by connective tissue containing scanty longitudinal bundles
of the smooth muscle cells. The external tunic of the ulnar vein is in 2-3
times thicker than both internal and internal tunics.
Veins Containing Considerable Amount
of the Smooth Muscle Cells
These veins are located
in the lower part of the body and legs, for example the femoral vein. The vein
internal tunic consists of 3 layers: endothelial, subendothelial, and elastic
fibers layers. Due to the internal tunic the venous valves (Fig. 100) are
formed. When the blood flows towards the heart, the valves are opened. In the
back flow of the blood the valves are closed. The subendothelial layer of the
femoral vein contains considerable amount of loose connective tissue and
longitudinal bands of the smooth muscle cells.
The middle tunic of the
vein is made up of the circular smooth muscle cells, collagen and elastic fibers
forming the framework of the vein wall.
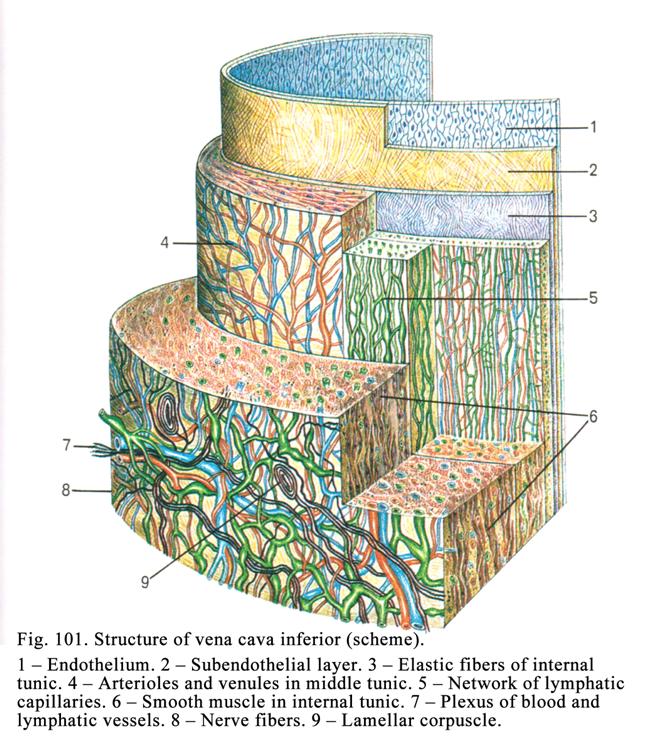
The external tunic of the femoral
vein is made up of the loose connective tissue and longitudinal bundles of the
smooth muscle cells. The smooth muscle cells of the vein wall promote the blood
flow towards the heart.
Inferior Vena Cava (Fig. 101)
The structure of the internal and
middle tunics of the vein is similar to those of the veins containing scanty or
moderate amount of the smooth muscle cells while the structure of its external
tunic is similar to that of the vein containing considerable amount of the
smooth muscle. Therefore the inferior vena cava may be referred to the veins
containing considerable amount of the smooth muscle cells. The external tunic
of the vena cava inferior is in 6-7 times thicker than both internal and middle
tunics. The smooth muscle cells contraction of the vein wall pushes the blood
towards the heart.
CHAPTER 14
LYMPHATIC VESSELS & THEART
The lymphatic vessels also
belong to the cardiovascular system along with the blood vessels and the heart.
Lymphatic Vessels
They are divided into 1)
the lymphocapillary vessels, 2) draining intraorganic and extraorganic
lymphatic vessels, and 3) the largest lymphatic vessels (thoracic duct and
right lymphatic duct). In addition the lymphatic vessels are divided into 1)
the fibrous lymphatic vessels and 2) the muscular lymphatic vessels.
The hemodynamic within
the lymphatic vessels is similar to that of the veins. The lymphatic vessels
have well developed external tunic, valves are formed due to the internal
tunic.
Lymphatic Capillary Vessels
The lymphatic capillary
vessels (Fig. 95, 7) are located in connective tissues where they form the
network. They are lodged near the blood capillary vessels. The lymphatic
capillary vessels have close anatomical and functional connection with the
blood capillaries. From the blood capillaries into the fundamental substance of
connective tissue necessary components pass. From the fundamental substance
metabolic products, pathologic constituents, and cancer cells enter the
lymphocapillary vessel. Blood and lymph capillaries have some differences. They
are: 1) lymphocapillary vessels have larger diameter, 2) their endothelial
cells in 3-4 times larger, than blood ones, 3) there are no basal membrane and
pericytes in the lymphocapillary vessel walls, and 4) the lymphocapillary
vessel end is blind.
The lymphocapillary
vessels drain into intraorganic or extraorganic lymphatic vessels.
The lymphocapillary
vessels functions are: 1) from the connective tissue fundamental substance
various constituents enter lymphocapillary vessels, they compose the lymph, 2)
metabolic products pass into the capillary, and 3) cancer cells enter
lymphocapillary vessels then the cells enter the blood stream, which carries
them to various organs.
Draining Intraorganic Lymphatic
Vessels
They are fibrous
lymphatic vessels (40 mm in
diameter). Their endothelial cells lie in the thin discontinuous membrane.
Under membrane there are collagen and elastic fibers running into the external
tunic. The fibrous lymphatic vessels may be referred to the postcapillary
vessels they also have valves. They provide the drainage function.
Draining Extraorganic Lymphatic
Vessels
They are larger than
intraorganic lymphatic vessels. They are referred to the muscular lymphatic
vessels. If the extraorganic lymphatic vessels are located in the body upper
part (face, neck, etc.) they have little smooth muscle cells. If the vessels
are located in the lower body part, they have more muscle cells.
Lymphatic Vessels of Middle Caliber
They are also referred
to the muscular lymphatic vessels. In their wall 3 tunics are seen: internal,
middle, and external ones. The internal tunic consists of 3 layers: endothelium
located on the thin basement membrane, subendothelial layer, containing
collagen and elastic fibers, running to different direction, and the plexus
made of elastic fibers.
The internal tunic provides formation of the valves of
lymphatic vessels. The cord of the valve cusps is the fibrous plate. In its
center there are some smooth muscle cells. Endothelial cells cover the plate.
The middle tunic of the vessels is made up of smooth
muscle cell bundles, arranging in circular and oblique directions, and loose
connective tissue.
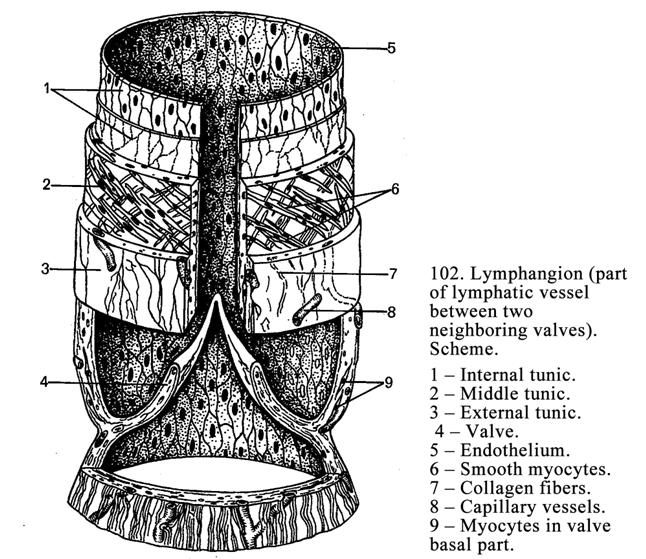
The external tunic of the vessels consists of the
connective tissue, containing collagen and elastic fibers, entering the
surrounding tissue.
The Segment Placed Between Two
Following Valves
This segment includes
the muscular collar, valve sinus wall, and the place where the valve cusps are
attached (Fig. 102).
The Largest Lymphatic Vessels
They are the thoracic
duct and the right lymphatic duct. Every tunic of these ducts contains smooth
muscle cells.
The Thoracic Duct
It drains lymph from
grater part of the body. Its wall structure is similar to that of the inferior
vena cava. Its internal tunic is made up of endothelial layer,
subendothelial layer, and the plexus of elastic fibers. Endothelial cells lie
on the thin discontinuous membrane. The subendothelial layer contains the stem
cells, smooth muscle cells, and collagen and elastic fibers arranging in
various directions. The internal tunic provides formation of 9 valves promoting
the lymph flow towards the neck veins.
The lymphatic vessel middle
tunic is made up of the circularly and obliquely directed smooth muscle
cells, collagen and elastic fibers passing in various directions.
On the diaphragm level
the external tunic of the thoracic duct is in 4 times thicker than that
of both internal and external tunics. The external tunic consists of loose
connective tissue and longitudinal bundles of the smooth muscle cells. The
thoracic duct drains into the neck vein. At this place the thoracic duct wall
thickness is in 2 times thicker than that of both internal and middle tunics.
Lymphatic System Functions
The lymphatic system
provides 1) drainage function (metabolic products, harmful substances,
bacteria, cancer cells pass into the lymphocapillary vessels), 2) bacteria,
toxins, and other harmful substances are removed from the lymph through
phagocytosis by macrophages in the lymph nodes, and 3) lymphocytes enter the
lymph from the lymph nodes. And the purified, consisting number of lymphocytes,
lymph come back into the blood stream i.e., the lymphatic system renews the
connective tissue fundamental substance and also renews the body internal
medium.
Blood Supply of the Vessel Wall
In the external tunics
of the walls of blood vessels and lymphatic vessels there are small arterial
and venous vessels (vas vasorum) forming capillary network within external and
middle tunics of the arteries and external, middle and internal tunics of the
veins. From the artery wall capillaries drain into the venules leaving the wall
close located to the arterioles. From the capillaries of the vein internal
tunics the deoxygenated blood passes into the vein lumen.
The blood supply of the
large lymphatic ducts differs from that of blood vessels. The venules, leaving
the walls of the lymphatic vessels, run separately from entering arterioles. In
the walls of the arterioles and venules there are no vas vasorum.
Regeneration of the Blood Vessels
after Damages
When the blood vessel
wall is damaged, the rapidly dividing endothelial cells cover the place in 24
hours. Regeneration of the smooth muscle cells is not rapidly provided because
the muscle cells divide more seldom. New myocytes are formed due to cell
division or conversion of myofibroblasts or pericytes into the smooth muscle
cells.
If the large blood
vessel is entirely ruptured, it cannot be renewed (the surgeon mast operate).
But supply of the tissue distally of the rupture may be recovered due to
collateral vessels and newly formed small blood vessels. For example, from the
walls of arteriole and venule endothelial buds arise and move towards each
other to fuse and form new capillary vessel.
The Nerve Supply of Blood Vessels
Nervous regulation of
the blood vessels is provided by efferent (sympathetic and parasympathetic)
nervous fibers and afferent (sensory) nervous fibers representing dendrites of
neurons of the spinal and cranial nervous ganglions. The efferent and afferent
nervous fibers surround the blood vessels to form nerve plexuses containing
single neurons and small ganglions.
The sensitive nervous
fibers are ended by receptors having complicated structure i.e., they are
multiform. It means that one receptor simultaneously comes in contact with the
arteriole, venule, arteriovenous anastomose, and connective tissue structures.
In the external tunic of the large blood vessels various receptors
(encapsulated nerve corpuscles and non-encapsulated tactile corpuscles) forming
large receptive area may be located.
The efferent nerve fibers are ended
by effectors (motor nervous endings). The sympathetic nervous fibers are the
axons of efferent neurons of sympathetic nervous ganglions. They end with
adrenergic nerve terminals.
The parasympathetic
nervous fibers are the axons of efferent neurons (Dogels cells of type I) of
the parasympathetic nervous ganglions. They are cholinergic nerve fibres ending
by cholinergic nerve terminals. If the sympathetic nerve fibers are excited,
blood vessels become shrinks. When the parasympathetic nervous fibers are
excited, the blood vessels are dilated.
The Blood Vessel Regulation by
Neurons and Endocrine Cells
This regulation is
characterized by sending the nervous impulse produced by excited neuron to the
single endocrine cells. The cells release hormonal factor influencing the blood
vessels.
The Endothelial Cell Regulation
Blood Flow
The endothelial cells
release factors regulating contraction of the smooth muscle cells of the vessel
wall. In addition endothelial cells release factor, inhibiting blood clot, and
factor stimulating the blood clot.
Age Dependent Alteration of the
Arteries
The arteries are
completely developed by the age of 30. By 40 years their structure is stabile.
After 40 the involution begins. In the wall of large arteries elastic fibers
and smooth muscle cells are destroyed while the collagen fibers grow to form
swells in the subendothelial layer. It results in deposition of cholesterol,
sulphate glycosaminoglycans, and salts so the artery sclerosis is formed and
the organ blood supply is destroyed. In persons older than 60-70 years in the
external tunic of the vessels longitudinal bundles of the smooth muscle cells
appear.
Age Dependent Alteration of the
Veins
The vein alteration is
similar to that of the artery. But in the veins alterations begin much earlier.
In the subendothelial layer of the femoral veins of the newborn and children of
1 year there are no longitudinal smooth muscle bundles. They appear when the
children begin to go. The diameter of veins and arteries is equal in little
children,but the vein diameter of the adult is in 2 times wider than in
arteries because the blood flow in veins is slower than in the arteries. It
provides the balance of the blood flow to and out of the heart. The vein wall
is thinner than that of the artery. It can be explained by hemodynamics in the
vein distinguishes from that of the artery i.e., the blood pressure and blood
flow is lower in the vein.
The Heart
The heart includes atria
and ventricles divided by the interventricular septum.
The Heart Development
The heart is derived from
the mesenchyme and internal visceral mesoderm of its cranial part on the 17th
day of the uterine life. Cardiac tubules, formed from the mesenchyme,
invaginate into the visceral mesoderm to form cardiac plates (left and right).
Then these places approach to each other by the trunk folds to form the
primitive heart consisting of two chambers. From the mesenchymal tubules the
endocardium is derived. From the external part of the cardiac plate the cardiac
myocytes, cardiac endocrine cells, and conducting cardiac cells are derived.
At the time of
differentiation cardiac myocytes assume columnar shape. The cells join by their
ends to form the intercalated discs. The cardiac myocytes constitute long chain
called cardiac myofiber. The developing cardiac myocyte includes myofibrils,
arranging longitudinally, mitochondria, smooth ER forming longitudinal and
transverse tubules.
The cardiac conducting
system of the embryo is formed during 2-4 months. The valves of the heart are
derived from the endocardium. The left atrioventricular valve is developed from
the endocardium fold in 1-2 months of the uterine life. Into the folds the
connective tissue grows to form the fibrous plate of the cusps of the valves.
The valve cusps are attached to the fibrous anulus.
The right
atrioventricular valve is formed from the fold of the endocardium including the
smooth muscle cells. Connective tissue grows from myocardium and epicardium
into this fold. Further in the right valve cusps the amount of the smooth
muscle cells becomes less. These cells persist only in the basal part of the
cusps.
On the 7th
weeks of the uterine life within the cardiac wall the intramural
(parasympathetic) ganglions are formed. The ganglion multipolar neuron
processes join with each other to form synapses.
The wall of the heart
consists of 3 tunics: 1) endocardium, 2) myocardium, and 3) epicardium.
The Endocardium
It lines the lumen wall
surface of the atria and ventricles. In different parts the endocardium has
different thickness. The endocardium is composed of 4 layers: 1) endothelial
layer, 2) subendothelial layer, 3) myoelastic layer, and 4) external connective
tissue layer. Thus, the cardiac
wall structure is
similar to that
of the muscular type vein i.e.,
the endocardial endothelium is similar to that of the vein the endocardium
subendothelial layer is similar to that of the vein the myocardium myoelastic
layer corresponds to the elastic fiber plexus and middle tunic of the vein and
endocardial connective tissue layer is similar to the vein external tunic. The
atrioventricular valves, the aortic valve, and the pulmonary artery valve are
formed from the endocardium.
The Left Atrioventricular Valve
It is made up of 2 cusps
(bicuspid valve). The valve cusp cord (Fig. 103) is the fibrous plate, containing
collagen and elastic fibers, a little amount of cells, and fundamental
substance. The cusps are attached to the fibrous anulus surrounding the valve.
The endothelial cells cover the cusps. The subendothelial layer is located
under the endothelial cells.
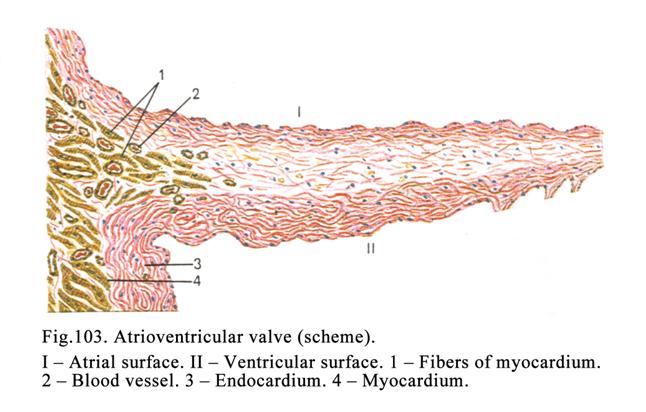
The Right Atrioventricular Valve
This valve is made up of
3 cusps (tricuspid valve). The atrial surface of the valve cusps (both left and
right) is smooth while the ventricular that is wrinkling because to the latter
the tendons of the papillary muscles are attached.
The Aortic and Pulmonary Artery
Valves
They are called the
semilunar valves. The valves are made up of 3 layers: 1) internal, 2) middle,
and 3) external layers.
The internal layer is made up of the endocardium,
consisting of the endothelium, subendothelial layer, which contains fibroblasts
supporting the endothelial cells. Deep to this layer the collagen fiber layer
and elastic fiber layer is located.
The middle layer is made up of the loose connective
tissue. The external layer is composed of the endothelium of the blood
vessels (aorta and pulmonary artery) and subendothelial lamina containing
collagen fibers entering here from the fibrous anulus.
The Myocardium
The myocardium consists
of myofibers, which are made up of cardiac myocytes.
Every cardiac myocyte is
cylinder shaped 50-120 mm in
length and 15-20 mm in
diameter. The places, where the cardiac myocytes join, are called the
intercalated discs. In the disc area there are desmosomes, nexuses, digiform
intercellular junctions, and places, where the actin filaments are attached.
In the center of the
cardiac myocytes there are 1-2 oval polyploid nuclei. In the cardiac myocytes
there are well developed mitochondria, smooth ER, and myofibrils but the rough
ER, Golgi complex, and lysosomes are scanty there. The acidophilic cytoplasm of
the cardiac myocytes contains the glycogen, lipids, and myoglobin. Myofibrils
of the cardiac myocytes consist of the actin (thin filaments) and myosin (thick
filaments) filaments. The actin filaments provide the isotropic bands (I
bands). Both the myosin filaments and the ends of actin filaments, entering
between the former, provide formation of the anisotropic bands (A bands).
Running across the middle of each I band there is a thin dark line called the Z
line (end of sarcomere). Running through the center of each A-band there is a
thin line called the M line. Between the ends of the actin filaments in the
A-band center there is a pale stripe called the pale zone (H band). The segment
of the myofibril between two Z lines is called the sarcomere. Opposite each
sarcomere there is the system of the smooth endoplasmic reticulum tubules
forming longitudinal and transverse tubules. The latter are called the lateral
cisterns. The tubule systems surround the myofibrils. Opposite the Z line the
sarcolemma of the cardiac myocyte is invaginated into the sarcoplasm to form
the T tube located between adjoining lateral cisterns.
The structure consisting
of two lateral cisterns and the T tube is called the triad. (It can be composed
of one lateral cistern and the T tube. It is called dyad).
On the lateral surface
of the cardiac myocytes there are some processes. They are attached to the
lateral surface of the adjacent cardiac myocytes to form anastomoses. Due to
the anastomoses the myocardium is an entire muscular network.
The cardiac muscle is
attached to the heart skeleton formed by the fibrous annuluses surrounding the
atrioventricular valves and valves of the aorta and pulmonary artery.
Atrial Cardiac Myocytes Releasing
Natriuretic Hormone
These cardiac myocytes
contain number of processes. In these myocytes myofibrils, smooth ER, T tubes,
and intercalated discs are poorly developed while the Golgi complex, rough ER,
and mitochondria are well developed. The atrial cardiac myocytes release the
natriuretic hormone. The hormone influences the special receptors of cells
perceiving for it (hormone). These receptors are located in the cardiac
myocytes of the heart ventricle, blood vessels, endocrine cells of the adrenal
cortex zone glomerulosa, and endocrine cells of the kidney. The natriuretic
hormone regulates the myocardium contraction, blood pressure, metabolism of
salts, and urinary excretion.
The Natriuretic Hormone Influence
Mechanism on the Target Cells
The target cell receptor
receives the natriuretic hormone to form the special complex. The latter
activates cyclic GMP, which influences the cell enzymes.
The Cardiac Conducting System
The system is composed
of the sinoatrial node, atrioventricular node, atrioventricular fascicle,
trunk, right and left bunds (Fig.104).
The Sinoatrial Node
In the center of the
node there are nodal (pacemaker) cells. The pacemaker cells are narrow,
rounded, oval or polygonal cells with one nucleus. Their poorly developed
myofibrils come in different directions, the smooth ER is inconspicuous, and
there are no intercalated discs and T tubes in their cytoplasm. The pacemaker
cells contain abundant mitochondria and glycogen granules. In the cytoplasm
there is free calcium due to it the cells are able to produce contractile
impulses rhythmically i.e., they response for pacemaker function.
Outside the pacemaker cells there
are transitional conductive myocytes. They are long narrow cells containing a
few parallel myofibrils. In these cells intercalated discs and T tubes are
inconspicuous. The transitional conductive myocytes provide the impulse
conduction to conductive cardiac myocytes or contractile cardiac myocytes.
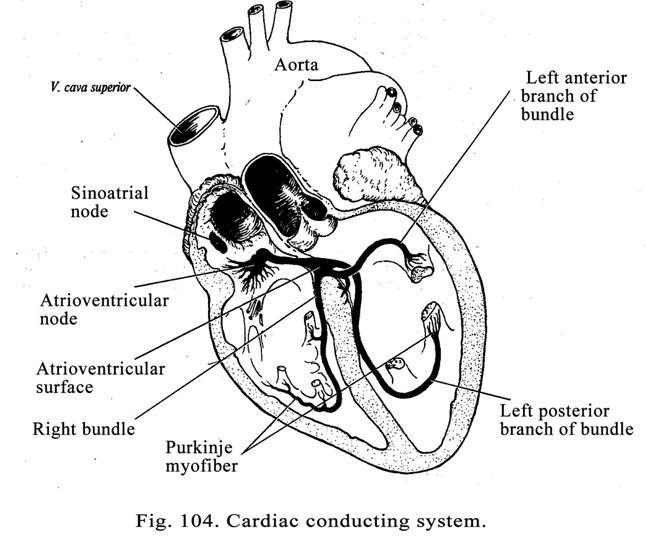
The Atrioventricular Node
The node includes a few
of the pacemaker cells, localized in the node center, and a number of the
transitional conductive myocytes in the node periphery. The atrioventricular
node functions are: 1) it produces impulses (30-40 per minute); 2) it delays
the impulses conduction from the sinoatrial node to the ventricular muscle for
a short period of time. At the same time the atriums contract thereafter the
impulse passes to ventricles and produce them contraction.
If the impulse cannot
passes from the synovial node to the atrioventricular node, the atria and
ventricles contract with different rate (the atria contract 60-80 times per
minute while the ventricles contract 30-40 times per minute). It is life
threatening condition.
Conductive Cardiac Myocytes
(Purkinje Cells)
The myocytes are located
in the trunk, right and left bundles and their brunches. These cells are of 50-120 mm in length and about 50 mm in diameter. They are often
localized deep in the endocardium but they can be found among myofibers. The
Purkinje cell, forming conductive cardiac myofiber, comes in contact with
papilla muscles. Due to this at the moment of the ventricular contraction the
papilla myofibers are strained, that prevents the valve cusp invagination into
the atrium.
The Heart Nerve Supply
The heart is supplied by
the sensory and efferent nerve fibers. The former are the neuron dendrites of
1) the spinal ganglions, 2) the vagal ganglion, and 3) the intramural ganglions
in the heart wall. The dendrites are terminated by the receptors.
The efferent nervous
fibers are the sympathetic and parasympathetic nervous fibers belonged to the
autonomic nervous system.
The sympathetic
reflex arc is the
chain, consisting of 3 neurons. The first neuron is in the spinal ganglion the
second neuron is in the lateral nucleus of the grey commissure the third neuron
is in the sympathetic trunk or celiac ganglion.
The Impulse Pathway through the
Sympathetic Arc
From the receptor the
impulse passes to the first neuron dendrite then to the axon of the neuron then
to the second neuron dendrite then to the axon of the second neuron, called the
preganglionic cholinergic myelinated nervous fiber, coming in contact with the
third neuron dendrite then to the third neuron axon, called the postganglionic
nonmyelinated adrenergic nervous fiber then to the conductive myocyte. When the
sympathetic fiber is activated, the heart contraction rate rises (becomes
rapid).
The parasympathetic
reflex arc consists
of 3 neurons. The first neuron is in the sensory ganglion of the vagus nerve the
second neuron is in vagal nucleus the third neuron is the intramural ganglion.
The Nervous Impulse Pathway Trough
the Parasympathetic Arc
From the receptor the
impulse passes to the first neuron dendrite then to the neuron axon then to the
second neuron dendrite then to the neuron axon, called the preganglionic
nervous myelinated fiber, then to the third neuron dendrite then to the neuron
axon, called the postganglionic cholinergic nonmyelinated nervous fiber then to
the conductive myocyte of the heart. When the parasympathetic nervous fiber is
activated, the heart contraction rate is slow.
The Epicardium
The external surface of
the myocardium is covered by the epicardium (or the visceral layer of the
serous pericardium). Both the epicardium and parietal serous pericardium are
composed of the connective tissue covered with flattened mesothelial cells on
its luminal surface. Between the epicardium and pericardium there is the
slit-like space (cavity) filled with some amount of fluid (lubricant). The epicardium
is derived from the visceral mesoderm while the pericardium is derived from the
parietal mesoderm. The connective tissue of the epicardium and pericardium
contains adipocytes.
Age Dependent Changes of the Heart
The development of the
heart includes 3 stages: 1) the differentiation stage, 2) the stabilization
stage, and 3) the involution stage.
The differentiation
stage begins in the
embryonic life. After birth the blood circulation undergoes alteration. Soon
after birth the heart oval window or the meatus (between the right and left
atriums) is closed. The duct, joining the aorta and pulmonary artery, is closed
too. It leads to blood flow low into the heart right ventricle as a result this
ventricle undergoes the physiological atrophy. Simultaneously the exertion of
the left ventricle is increased that results in its physiological hypertrophy.
The cell cytoplasm mass,
amount and size of myofibrils of the typical cardiac myocytes become larger.
The cardiac myofibers are surrounded by the thin layer of loose connective
tissue.
The stabilization
stage begins at the
age of 20 and ends at the age of 40.
The involution stage begins after 40. The myocyte
thickness becomes less due to the decrease of the thickness and amount of
cardiac cell myofibrils. The layer of connective tissue, surrounding the
cardiac myofibers, becomes thicker. The sympathetic nervous fiber amount
becomes less while that of the parasympathetic nervous fibers does not alter.
It leads to the decrease of the rate and strength of the cardiac muscle
contraction. After age 70 number of the parasympathetic nervous fibers
decreases. In the blood vessels of the heart the sclerosis is formed, which
leads to narrowing of the arterial lumen, and consequently to blood flow
reducing and myocardium blood supply decreasing. This condition is called
ischemic disease.
The Myocardium Blood Supply
The blood supply of the
myocardium is provided by the coronary arteries arising from the aorta. The
coronary arteries are the muscular type arteries. But in their subendothelial
layer there are longitudinal bands of the smooth muscle cells. The coronary
arteries branch to form capillary network. The capillary vessels enter venules
and coronary veins draining into the right atrium or the venous sinus.
The Myocardium Regeneration
The regeneration of the
cardiac myocytes is seen only in children of 1-2 years when the myocytes of the
heart are able to divide. In the adult destroyed cardiac myocytes are replaced
by connective tissue.
CHAPTER 15
ENDOCRINE SYSTEM CENTRAL ORGANS
Along with the autonomic
nervous system the endocrine organs coordinate and control the metabolic
activities and the internal environment of the body. But as for the endocrine
system it mainly coordinates more general processes: metabolism, body growth,
and reproduction of gametes. The endocrine system includes the glands releasing
their secretions (hormones) into the blood or lymphatic vessels. Therefore the
endocrine glands are more vascular, than exocrine ones. In addition in the
endocrine glands the gland ducts are absent.
The capillary network of
the endocrine glands has 3 features: 1) they include sinusoids, 2) in the
sinusoids there are fenestrated endothelial cells, and 3) around of the
capillary vessels there are perivascular spaces.
Chemical Nature of Hormones
Hormones are
predominantly amino acid derivatives, small peptides, and proteins. Lipid
derivatives (steroids) are released by the sex glands and adrenal cortex.
Some hormones are released
only by one of the gland (thyroxin is released by the thyroid gland) other
hormones are released by some glands (insulin is released by the pancreas, the
parotid gland, and the thymus). There are single cells releasing some hormones
(G-cells of the stomach release gastrin and encephalin).
Hormone influences the
cell that bears special receptors that are sensitive only to it. They are
called target cells.
The Hormone Influence Mechanism on
the Target Cell
When the receptor
receives the hormone, the special complex, consisting of the receptor and
hormone, is formed. The complex activates adenylate cyclase, which induces the
cAMP synthesis. The latter stimulates the cell enzyme activity.
The Endocrine System and Nervous
System Interaction
The endocrine system is
regulated by the nervous system. 2. The nervous and endocrine cells produce
biologically active substances (endocrine cells produce hormones wile nervous
cells produce neurotransmitters).
Classification of the Endocrine
Organs
The endocrine organs are
divided into: A) central endocrine organs (hypothalamus, epiphysis cerebry, and
hypophysis). B) Periphery endocrine organs. The latter are further divided
into: 1) the endocrine glands (thyroid gland, parathyroid glands, and
suprarenal or adrenal glands, 2) the groups of endocrine cells located in the
organs with other functions (pancreas, placenta, gametes), and 3) the isolated
endocrine cells, distributed in different organs, forming the diffuse
endocrine system. The diffuse endocrine
system is further divided into 1) the cells having neurogenous origin. All
these cells absorb precursor of amines and andergo them decarboxylation to form
and secret smoll peptides and neuroamines. The cells of this system contain
spherical membrane bound granules with a dense core. There is the
electronlucent halo around the dense core. These cells are, usually stained by
metal salts. They are, therefore, included in the APUD (Amine Precursor Uptake
and Decarboxylation) cell system. 2. There are also isolated sells, which are
not included in the APUD cell system (interstitial cells of gametes releasing
steroids).
Depend on the functional
features the endocrine organs are divided into 1) the neuroendocrine organs
(nuclei of hypothalamus) releasing hormones, 2) the organs, which no hormones
are synthesized but they receive them from the hypothalamus (the median
eminence and the neurohypophysis), 3) the central organ (adenohypophysis)
regulating the peripheral endocrine masses, and 4) the peripheral endocrine
organs dividing into a) hypophysis dependent organs (thyroid gland, adrenal
cortex, and gonads) and b) hypophysis in-dependent organs (parathyroid glands,
parafollicular cells within thyroid gland, and medulla of the suprarenal
glands).
The Hypothalamus
The hypothalamus is
divided into the anterior hypothalamus, the middle hypothalamus, and the
posterior hypothalamus. The hypothalamus is closely connected with the
hypophysis (Fig. 105) by two tracts (systems): 1) the tract connecting the
middle hypothalamus with the adenohypophysis and 2) the tract connecting the
anterior hypothalamus with the posterior lobe of the hypophysis
(neurohypophysis).
Every tract has its own
organ in which hormones are not synthesized called neurohemal organ. In the
first system (tract) such organ is the median eminence in the second system
such organ is the neurohypophysis.
The features of
neurohemal organ:
1) the capillary network is abundant; 2) the capillary vessels are connected
with the neurosecretory neuron axons by the neurovascular synapses, and 3) these
organs are able to accumulate nervous hormones.
The Hypothalamus Neurosecretory
Nuclei
In the hypothalamus
there are 30 pairs of nuclei but we consider only 8 pairs of them. Some nuclei
contain large cholinergic neurosecretory cells other nuclei contain small
adrenergic neurosecretory cells. Both large and small cells are able to
multiply.
Nuclei of the anterior
hypothalamus are represented by 2 pairs: 1) the supraoptic nuclei and 2) the
paraventricular nuclei.
These nuclei contain the
large cholinergic neurosecretory cells secreting peptides and acetylcholine. In
addition the paraventricular nuclei contain the small adrenergic neurosecretory
cells. The large cholinergic and small adrenergic neurosecretory cells do not
only release the neurosecretory hormones but also generate and conduct nervous
impulses.
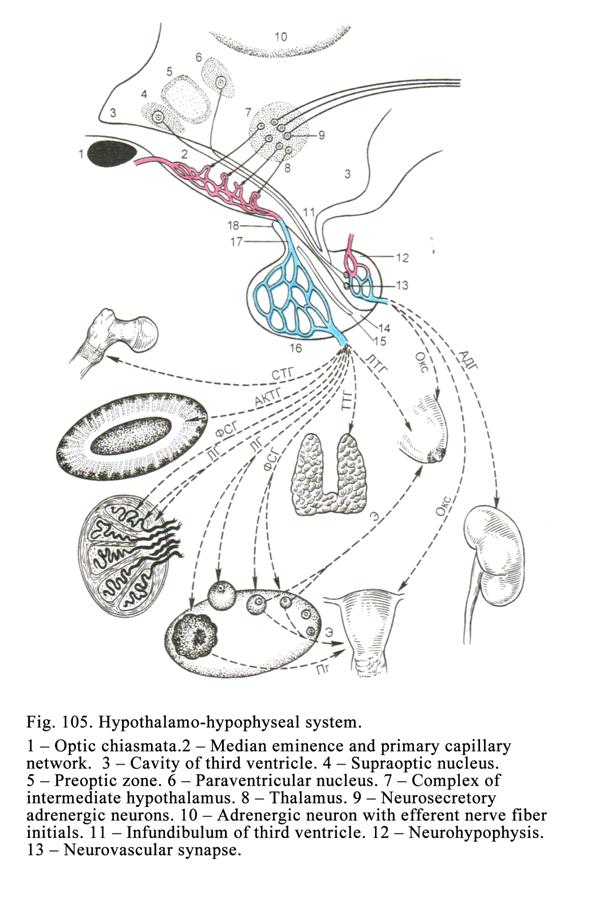
The large neurosecretory
neurons contain dense secretory granules and release 2 hormones: vasopressin
(antidiuretic hormone) and oxytocin. Oxytocin is synthesized predominantly by
the paraventricular nuclei.
Vasopressin Properties
Vasopressin provides: 1)
constriction of the blood vessels and 2) stimulation of the water reabsorption
from the renal tubules into the blood capillary.
Oxytocin Properties
Oxytocin stimulate: 1)
contraction of the myoepithelial cells of the breast terminal regions as a
result the milk releasing becomes more intensive, 2) contraction of the uterine
muscle, and 3) contraction of the smooth muscle of the male reproductive organ
ducts.
In the cell bodies of
the neurosecretory neurons and their axons of the supraoptic and
paraventricular nuclei there are granules containing vasopressin and oxytocin.
These granules pass down along the axons to the neurohypophysis and deposit
near the blood capillary vessels. These deposits are called the accumulating
neurosecretory bodies.
The Nuclei of the Middle
Hypothalamus
The middle hypothalamus
contains 6 pairs of the neurosecretory nuclei: 1) the arcuate (or infundibular)
nuclei, 2) the ventral medial nuclei, 3) the dorsal medial nuclei, 4) the
suprachiasmatic nuclei, 5) the pre optic zone, and 6) the surrounding ventricle
grey substance.
The largest of them are
the arcuate nucleus and the ventral medial nucleus. Every nucleus of the middle
hypothalamus contains the small adrenergic neurosecretory cells, which are able
to multiply, produce and conduct nervous impulse. They contain dense granules
containing releasing hormones. These hormones are divided into the stimulating
releasing hormones (liberins) and inhibiting ones (statins).
The
releasing hormones influence the adenohypophysis. The exciting releasing
hormones (liberins) stimulate the adenohypophysis function while inhibiting
hormones (statins) inhibit it. Every releasing hormone influences the certain
cell of the adenohypophysis. For example, the thyroid releasing hormone
(exciting or inhibiting) influences the cells, producing the thyrotropin
(thyrotropic hormone), influencing the thyroid gland; the gonad releasing
hormone influences the cells, producing the gonadotropic hormone, influencing
the gonads, etc.
The Hypothalamus Regulation of the
function of Peripheral Glands
There are 2 pathways of
the regulation: 1) through the hypophysis and 2) the pathway other than
hypophysis.
Regulation through the Hypophysis
This pathway begins from
the nuclei of the hypothalamus. The neurosecretory cells of the nuclei secrete
releasing hormones, which pass through the capillary vessels of the eminence
towards the adenohypophysis to provide discharging tropic hormones (thyrotropin,
gonadotropic hormone etc.). The tropic hormones enter the blood capillary
vessels of the adenohypophysis. Blood carries these hormones towards the
peripheral endocrine glands. The hormones stimulate the glands functions (Fig
105).
The Pathways Other Than Through
Hypophysis
There are 3 species of
the pathways. The first is the
sympathetic or parasympathetic pathway (through the sympathetic or
parasympathetic nerve fibers). The hypothalamus is the regulating center of
the autonomic nerve system. The sympathetic and parasympathetic nerve fibers,
arising from the hypothalamus, reach the peripheral endocrine glands and
influence them.
The second is called the neuro-humoral pathway. For example, the small adrenergic
neuron of the paraventricular nucleus sends its axon to the anterior lobe of
the hypophysis. The axon acts on the corticotropic cell, which discharges
corticotropic hormone (ACTH), stimulating the adrenal cortex. Latter releases
cortisol, inhibiting the insulin releasing from the pancreas.
The third pathway involves immune cells. For example, the macrophage releases the
interleukin 1 (IL 1) entering the blood vessel. Blood carries IL 1 to the
paraventricular nucleus, which discharges releasing hormone (the hormone
stimulating the corticotropic cells). The hormone reaches the hypophysis
anterior lobe, which secretes ACTH. The latter influences the adrenal cortex,
which releases cortisol reaching the macrophage and inhibiting it as a result
the releasing of IL1 by macrophage is inhibited.
There are 3 additional
pathways of the peripheral gland regulation. The first additional pathway depends on the hormone level in the blood.
If the hormone level is low, the gland releases corresponding hormone. If the
hormone level is high, the releasing of the corresponding hormone is inhibited.
The second additional
pathway depends on the effect produced by the certain hormone. For example, the
parathyroid hormone provides the high level of calcium in the blood. The high
blood calcium level is the effect provided by the parathyroid hormone. If the level of calcium in the blood is high,
the release of the parathyroid hormone is inhibited. If the level
of calcium in the blood is low, the parathyroid hormone is released from the
glands.
The third additional pathway depends on the interaction between the thyroid
gland cell receptors and antibodies, stimulating secretion of thyroid hormone
from the thyroid gland. For example, receptors of the thyroid gland cells
receive these antibodies and hold them for some months as a result the thyroid
gland function is also active for some months.
The Hypophysis (Fig. 105)
It is composed of 4
parts: 1) the pars anterior (distal region), 2) the pars intermedia
(intermediate region), 3) the pars tuberalis (these 3 parts are collectively
referred to the adenohypophysis), and 4) the posterior lobe (neurohypophysis).
The Hypophysis Development
It is derived from the
oral cavity epithelium and the brain third ventricle fundus on the 5th
week of the embryonic life. The epithelium of the oral cavity upper part
invaginates to form pocket. The pocket grows toward the third ventricle of the
brain. The ventricle low part is called the infundibulum. When the pocket
reaches the infundibulum, the hypophysis is formed. The distal region of the hypophysis
is derived from the pocket anterior wall. The intermedia part is derived from
the pocket posterior wall. The posterior lobe is derived from the infundibulum
of the brain third ventricle.
The Hypophysis Distal Region
The distal region of the
hypophysis is covered by the connective tissue capsule, from which the
connective tissue enters between the parenchymal cells to form the stroma. In the stroma the blood and
lymphatic vessels are seen. Between the stroma there are clusters of the
parenchymal cells.
Classification of the Distal Region
Parenchymal Cells
The parenchymal cells
are classified into 1) the chromophil cells and 2) the chromophobe cells. The
chromophil cells are called so because they contain granules, which can be
stained by the dye. In the chromophobe cells there are no granules therefore
their cytoplasm cannot be stained by the dye (Fig. 106. 1).
Classification of the Chromophil
Cells
The chromophil cells are further
divided into the basophil cells and acidophil cells. In the anterior lobe there
are cells, which refer neither to the acidophil or basophil cells. They are the
corticotroph cells. Basophil cells contain granules staining by the basic dyes
while those of the acidophil cells staining by the acid dyes.
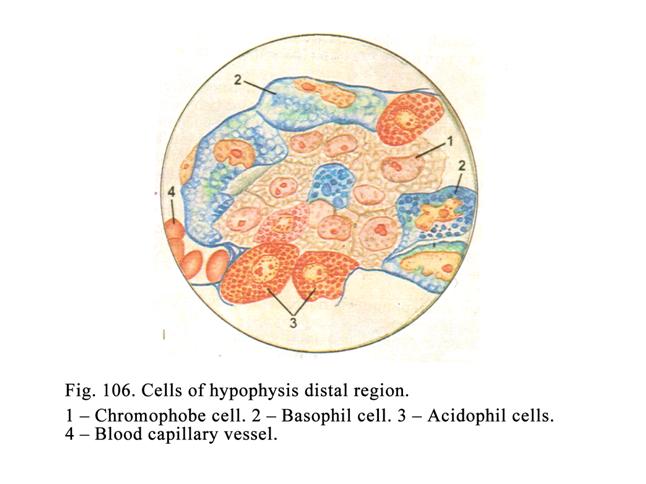
Basophil Cells
There are 4-10% of the
basophil cells in the anterior lobe. They are divided into the gonadotroph and
thyrotroph cells (Fig. 106. 2. Fig.107. I & II)
Gonadotroph large
round cells (Fig. 107. I) includ round nucleus, pushed to the cell periphery by the macula located
in the cell center. In the macula there are the Golgi complex and cytocenter.
In the cell cytoplasm the abundant rough ER, mitochondria, Golgi complex and
basophil granules (200-300 nm in diameter) are seen. The granules contain
glycoproteins stained by aldehyde fuchsin
The gonadotroph cells
produce two hormones: 1) the follicle stimulating hormone (FSH) and 2) the
luteinizing hormone (LH).
The follicle
stimulating hormone
of the male influences the initial stage of the spermatogenesis while in the
female it influences the follicle growth and release of estrogen by the ovary.
The luteinizing
hormone in the male
stimulates testis to release testosterone while in the female it stimulates the
corpus luteum to develop and realizing hormne progesterone.
According to some
investigators two classes of the gonadotroph hormones are produced by different
cells, but other workers hold that the same cells can produce both hormones.
Castratic cells (Fig.
107. VII) appear in
the hypophysis distal region when gonads produce insufficient amount of sex
hormones duo to some gonadotropic cells undergo hypertrophy to produce the
gonadotroph hormones and to stimulate the gonad function.
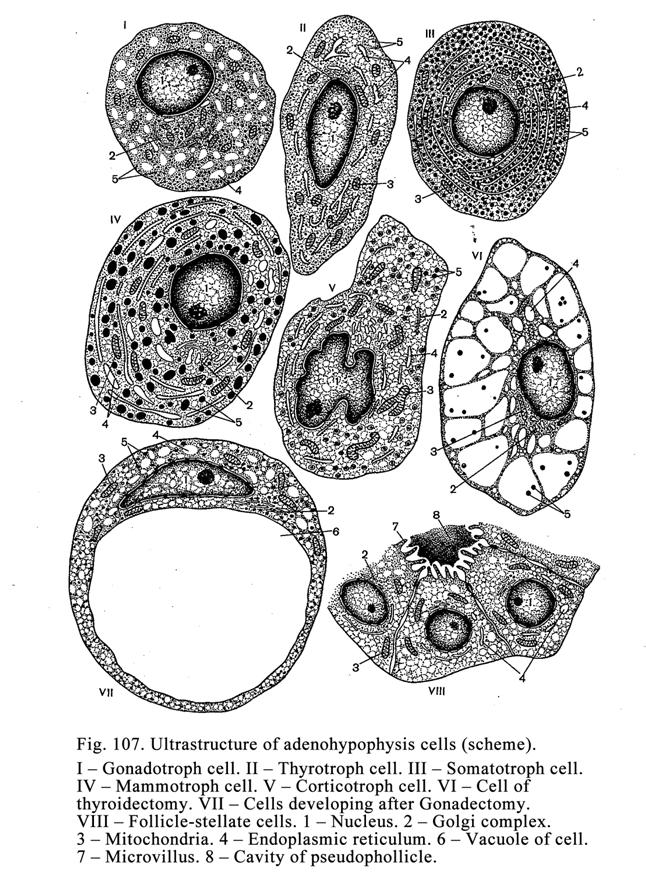
Thyrotroph cells (Fig. 107 II) have oval or
elongated shape, contain oval nuclei. In their cytoplasm there are prominent
Golgi complex, rough ER, mitochondria, and basophil granules (80-150 nm in
diameter) staining by aldehyde fuchsin. Releasing hormones discharged by the
hypothalamus influence thyrotroph cells. As a result cells secret the
thyrotroph hormone stimulating the thyroid gland function.
Thyroectomy cells (Fig. 107 VI) appear in the
hypophysis when the thyroid gland function is decreased. At the same time in
the thyroid cells the rough ER undergoes hypertrophy duo to this the ER
cisterns become wide, the discharge of the thyrotroph hormone is stimulated and
the thyroid gland function is increased. These cells assume spongy appearance.
Corticotroph cells
(Fig. 107 V) are
irregular shaped, have segmented nuclei. They not belong to basophil or
acidophil cells. Their cell cytoplasm contains a few organelles. The releasing
hormones of the hypothalamus stimulate these cells. They discharge the
corticotropic hormone (ACTH) stimulating the adrenal cortex function.
Acidophil Cells (Fig. 106. 3)
The amount of these
cells is 30-40%. They are divided into 1) the somatotroph cells and 2) the
mammotroph cells. Both types of cells are round shaped. They contain oval or
round nucleus located in the cell center. In the cell there are prominent Golgi
complex, rough ER, mitochondria, and acidophil granules.
The somatotroph cells (Fig. 107 III) granules are oval or
round shaped (400-500 nm in diameter). The cells produce somatotropic hormone.
This hormone stimulates the body growth (particularly before puberty). If there
is somatotroph cells hypertrophy in adult, the disease called acromegaly
results.
The mammotroph cells (Fig. 107 IV) contain elongated
granules. The granule length is 500-600 nm in the pregnant woman. The granule
length in adult female is, usually, about 200 nm in diameter. The mammotroph
cells release the mammotropic hormone stimulating 1) the growth of the female
mammary glands in pregnancy and 2) mammary gland loctation and the corpus
luteum growth and its function activity.
Chromophobe Cells (Fig. 106. 1)
The amount of these
cells is about 60%. The size of the cells is less than chromophil cells. In
these cells there are no granules therefore their cytoplasm cannot be stained.
The chromophobe cells are divided into 4 groups: 1) undifferentiated cells
(stem cells), 2) the cells, which begin to differentiate but this process is
not terminated, 3) the chromophil cells releasing their secretory granules
therefore their size is small and their cytoplasm cannot be stained, and 4)
star like cells having long processes. The groups of these cells can form
follicles (Fig. 107 VIII) filled with colloid. They are called pseudofollicles.
Intermediate Region (Fig. 105. 14)
It consists of some layers
of the epithelial cells. The intermediate region is located between the
anterior lobe (distal region) and the posterior lobe of the hypophysis. The
intermediate region contains pseudofollicles filled with colloid. This lobe
produces 1) melanocyte stimulating hormone and 2) hormone regulating the lipid
metabolism.
The Tuberal Region
The tuberal region (Fig.
105. 16) is the part of the adenohypophysis. It is continuous with the distal
region. The tuberal region contains the cuboidal epithelial cells. Its function
is unknown.
The Hypophysis Cerebry Blood Supply
This system begins from the inferior
hypophyseal artery which branches to form the primary capillary network (Fig.
105. 2) located in the mediate eminence. From this network the portal veins (Fig.
105. 17) arise. These vessels descend through the infundibular stalk to the
distal region (Fig. 105. 16) to form there the second set of the capillaries
draining into the hypophyseal veins (Fig. 105).
This arrangement is
referred to the hypothalamo-hypophyseal portal system. Capillary vessels of the
second set are located between the portal veins and the hypophyseal veins to
form the wonderful set.
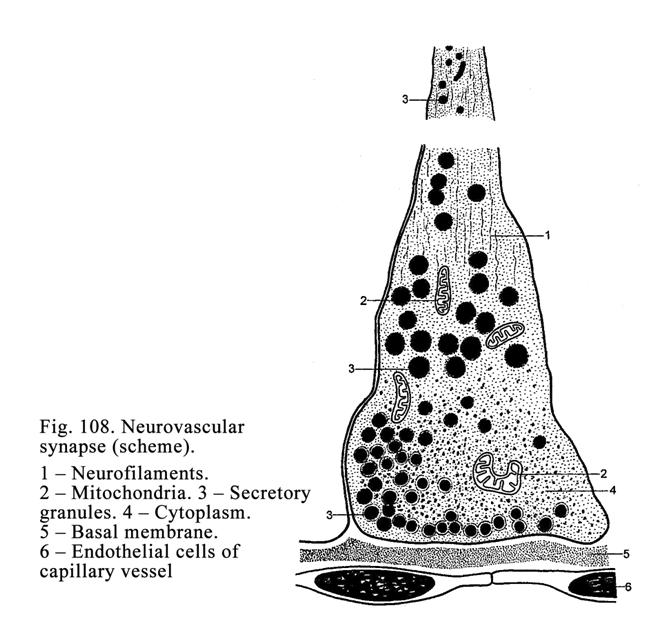
The Hypothalamo-hypophyseal System
Signification
The neurosecretory cell axons
conduct releasing hormones from the middle hypothalamus to the median eminence.
These axons terminate on the primary network of capillaries to form the
neurovascular synapses (Fig. 105. 13 & Fig. 108), through which releasing
hormones pass from the axons to the capillaries. Then the capillary blood
carries the releasing hormones through the portal veins to the second capillary
plexus (set) of the distal region of the hypophysis. There the parenchymal cell
receptors receive the releasing hormones to produce special hormones
(thyrotroph cells produce thyrotropic hormone, corticotroph cells produce ACTH
etc.). After that these hormones are carried by the blood to the corresponding
peripheral glands to stimulate them (the thyrotropic hormone stimulates the
thyroid gland, ACTH stimulates the adrenal cortex of the suprarenal glands
etc.).
The Posterior Lobe (Neurohypophysis)
Glial cells, called the
pituicytes and numerous of nonmyelinated nerve fibers, which are the axons of
neurons, located in the hypothalamus (supraoptic and paraventricular nuclei)
represent the lobe (Fig. 105.12). Through these axons vasopressin and oxytocin
pass to the neurohypophysis from the supraoptic and paraventricular nuclei.
Vasopressin and oxytocin are accumulated at the capillaries to form the
accumulating neurosecretory bodies. When it is necessary these hormones enter
the blood vessels from the bodies.
The Pineal Gland (Epiphysis)
The epiphysis is derived
from the two outgrowths (epiphiseal outgrowth and subcomissural organ) of the
brain third ventricle. The connective tissue capsule covers the epiphysis. From
the capsule the connective tissue cords arise, which divide the gland into the
lobules. The connective tissue cords or septa form the epiphysis stroma.
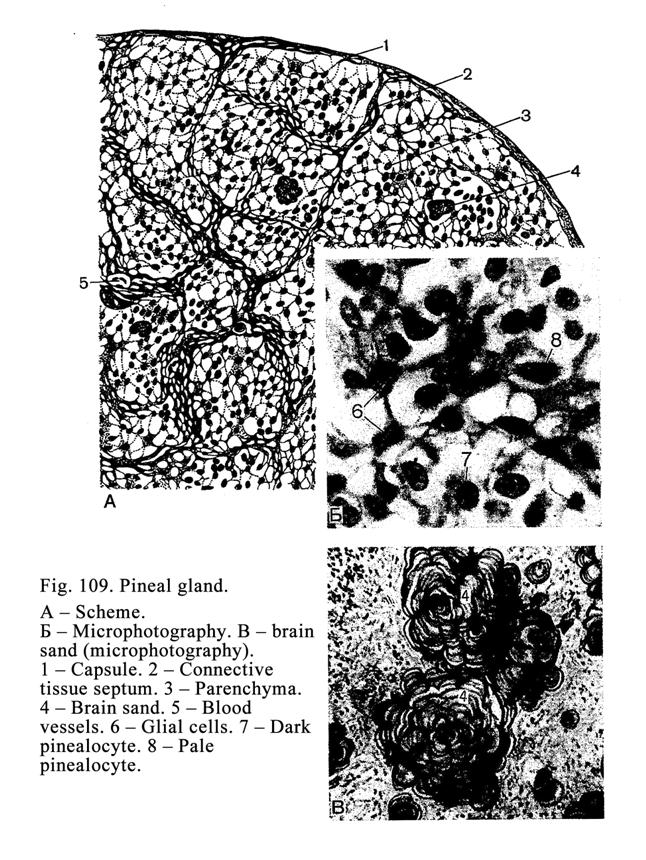
The epiphysis (Fig. 109)
parenchyma is made up of 2 types of cells: 1) sustaining cells and 2)
pinealocytes. The pinealocytes are divided into 2 types: 1) pale pinealocytes
and 2) dark pinealocytes. In the both pale and dark pinealocytes there are
well-developed mitochondria, rough ER, and Golgi complex. Their nuclei are
large and round. From the pinealocyte processes arise, which terminate on the
peripheral capillaries and surround the lobules. The process terminals are
thickened. In the processes and bodies of the pinealocytes there are secretory
granules.
The epiphysis provides
some functions. 1. The epiphysis regulates rhythmic processes. For example, one
regulates the circadian rhythm i.e., it may act as a kind of biologic clock
(depend on light or dark period of day). The pathway from the retina to the
epiphysis includes the hypothalamus and sympathetic nerves. Impulses, traveling
from the retina, influence the suprachiasmatic nucleus of the brain. The latter
influences the paraventricular nucleus of the hypothalamus. The neuron axons of
this nucleus reach the lateral nucleus of the spinal cord grey commissure. From
this nucleus nerve fibers run towards the sympathetic ganglion. From the
sympathetic ganglion nerve fibers reach the epiphysis cerebry. The epiphysis
also regulates the sex cycle of the female. 2. The epiphysis inhibits the
reproductive organ maturation. In the day-time the pinealocytes synthesize
hormone serotonin converting in melatonin. The latter inhibits discharge of the
releasing hormone from the hypothalamus stimulating the secretion of the
luteinizing hormone from the hypophysis necessary for the gonad function
activation. In addition the epiphysis produces special hormone inhibiting the
gonadotropic function of the hypophysis. 3. The epiphysis produces hormone
regulating the potassium blood level. 4. It produces the hormone constricting
the blood vessels. 5. This gland discharges the thyrotropic hormone and some
releasing hormones. 6.
The epiphysis cerebry
releases hormone stimulating aldosterone secretion from the
adrenal cortex. The epiphysis produces about 40 hormones.
Age Dependent Changes of the
Epiphysis
The epiphysis maturates
at the age of 6. After 30 the epiphysis undergoes involution. In the epiphysis
lobules the calcium carbonate deposits to form the brain sand (or corpora
arenacea). The brain sand deposits during all the human life (from the
childhood to the old age).
CHAPTER 16
THE PERIPHERAL ENDOCRINE ORGANS
In the human body there
are 3 peripheral endocrine glands: 1) the thyroid gland, 2) the parathyroid
glands, and 3) the adrenal (suprarenal) glands. In addition there is the
peripheral diffuse endocrine system.
The Thyroid Gland
The gland is located
near the shied-like cartilage.
The Thyroid Gland Development
The bud of thyroid gland
appears on the fourth week of embryonic life as the outgrowth of the pharynx
near the first and second branchial pouches. The outgrowth reaches the space
between the third and fourth branchial pouches. Here the outgrowth becomes
thick and bifurcated. At the same time the outgrowth is similar to the exocrine
gland i.e., its distal end resembles the exocrine gland terminal region while
its proximal end resembles the exocrine gland duct. At last the exocrine gland
duct is dissolved. Simultaneously on the tongue radix the cecal foramen of the
tongue is seen. The left and right parts of the distal end (terminal region)
are joined.
Sometimes the gland
duct has not dissolved and remains after childbirth. In this case the surgeon
must remove the gland duct. In the persisting distal end the clusters are
formed from the epithelial cells. These clusters are converted into the
follicles. At the same time the nerve crest cells enter the distal end. These
cells undergo differentiation to form the parafollicular cells. The surrounding
mesenchymal cells form the connective tissue capsule around the thyroid gland
bud. From the capsule the connective tissue septa arise to form the thyroid
gland stroma. Blood vessels and nerves enter the septa.
The Thyroid Gland Structure
The thyroid gland (Fig. 110) is
composed of two lobes joined by an isthmus.
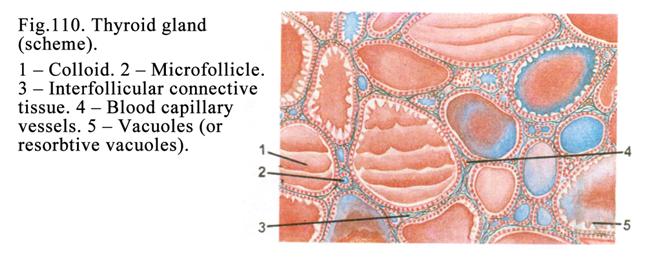
The connective issue capsule covers
the gland. From the capsule the connective tissue trabeculae arise into the
parenchyma. The trabeculae divide the gland into the lobules. The loose
connective tissue represents the gland stroma.
The Thyroid Gland Follicles
The follicle (Fig. 110)
is the functional and structural unit of the thyroid gland. The follicle has
round or oval shape. It may also be star shaped. Between the follicles there
are thin layers of connective tissue containing collagen and elastic fibers,
fundamental substance, fibroblasts, macrophages, mast cells, and plasma cells.
Round the follicles there are capillaries and nerve fibers.
Between the follicles
there are clusters of the epithelial cells called the follicular cell islets.
The epithelial cells located on the basal membrane line the internal surface of
the follicle wall. The follicle cavity is filled with colloid having fluid,
semifluid or thick consistence.
The follicular cells are
arranged in one layer and line the follicular wall internal surface. The free
surface of the follicular cells directs towards the follicle center, their
basal end are placed on the basal membrane.
The structure of the
follicular cells (Fig. 111) depends on the functional condition of the thyroid
gland. Normally (at the average level of activity) the cells are
cuboidal their free surface bears some microvilli. Desmosomes and denticulate
intercellular junction join the follicular cells. Near the apical part tight
junction closing intercellular spaces joins these cells. In the cell cytoplasm
there are conspicuous rough ER, Golgi complex, mitochondria, lysosomes, and
peroxisomes, containing enzymes, stimulating synthesis of the thyroglobulin,
modification of the latter within the Golgi complex, and oxidation of iodides
to form iodine atoms. Round cell nuclei are located in the center of the cells.
The colloid has semifluid consistence.
When the cells are highly active
they become columnar their free surfaces bear a number of microvilli and
pseudopodia. The colloid of the follicle becomes liquid. The fluid includes the
resorption vacuoles.
When the cells are
inactivated they
become flat. The follicle becomes large. The colloid becomes thicker.
The Secretory Cycle of the Follicle
The synthesis and
releasing of thyroid hormone is made of two phases: 1) productive phase and 2)
releasing phase.
The productive phase is
characterized by that the water, iodine ions, amino acid tyrosine,
carbohydrates, and other substances entrance the follicular cells and reach the
rough ER where the thyroglobulin is synthesized. Then the productions are
packed into secretory vesicles in the Golgi complex region. The vesicles travel
to the follicular cell luminal surface where the latter releases its product
(thyroglobulin) into the
follicular cavity by
the xocytosis. Simultaneously
iodine ions release the follicular cells luminal surface. There they oxidize to
form iodine atoms, which are combined with one thyroglobulin molecule to form
monoiodotyrosyl. When two iodine atoms are combined with one thyroglobulin
molecule, diiodotyrosyl is formed. When two iodine atoms are combined with one
monoiodotyrosyl the triiodothyronine is formed. If two iodine atoms are
combined with one diiodotyrosyl the tetraiodothyronine (thyroxin) is formed.
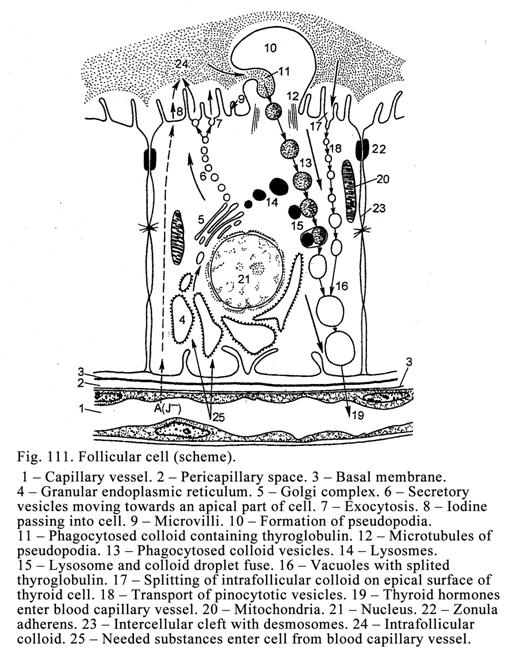
The releasing phase can
have some manners depend on the duration of the activity of the gland.
Normally (at the
average level activity) or in the increased activity of the gland for a long
period of time releasing of the secretion is as follows. On the apical surface of the
follicular cells the thyroglobulin splits due to this the triiodothyronine and
thyroxin become free. Then these hormones enter the follicular cells due to the
pinocytosis and pass to the capillary bed.
If the short-term
hyperfunction on the luminal surface of the follicular cells the amount of the
microvilli and pseudopodia is increased. The colloid is thinned. The follicular
cells engulf the colloid particles, wich are splited by the lysosomal enzymes
of the cells to form free triiodothyronine, thyroxin, diiodotyrosyl, and
monoiodotyrosyl. Triiodothyronine and thyroxin pass into the capillary network
Monoiodotyrosyl and diiodotyrosyl are splited so iodine atoms are free. They
are used for the synthesis of new hormones containing iodine
The Parafollicular Cells
The parafollicular cells
are derived from the nerve crest. They are located among the follicular cells
of the follicular wall or between the follicles (in the interfollicular
islets). The parafollicular cells are placed in the follicular wall are
triangular with oval eccentric nuclei. They are larger than follicular cells
but they do not reach the lumen. In the follicular cells there are granules
staining by silver or osmium therefore they are called argentophil or osmiophil
granules. In the cells there are well developed rough ER, Golgi complex, and
mitochondria.
The parafollicular cells
are divided into 2 groups. The first group of cells contains small granules
deep stained by osmium. They release thyrocalcitonin, which decreases the blood
calcium level. The second group of cells contains large granules slightly
stained by osmium. They release somatostatin, inhibiting synthesis of the
proteins in other cells. In addition to the somatostatin they release
noradrenalin and serotonin.
The Follicular Cell Function
Regulation
The hypothalamus and
hypophysis cerebry, blood level hormone, autonomic nervous system, and
epiphysis cerebry, releasing 2 hormones (hormone, stimulating distal region of
the hypophysis, and thyrotropic hormone stimulating the thyroid gland function)
regulate the follicular cell function.
Manners of the
thyroid gland regulation are as follows. The hypothalamus produces releasing hormones traveling into
the distal region of the hypophysis. These hormones (liberins) stimulate the
synthesis and secretion of the thyrotropic hormones, which enter the blood. The
latter carries them to the thyroid gland and stimulates the function of its
cells. If the hypothalamus discharges statins, inhibiting thyrotropic hormone
secretion, the thyroid gland function is terminated.
The activity of the
thyroid gland function depends on the blood level thyroid hormone. If
the level of this hormone is low, the follicular cells of the thyroid gland are
active. If the level of the thyroid hormone is high, they are inactive.
Regulation of the
activity of the follicular cells depends on the sympathetic and parasympathetic
nerve fibers condition. If the sympathetic nerve fibers are excited, the
follicular cells of thyroid gland are active. If the parasympathetic nerve
fibers are excited, those are inactive. But the autonomic nervous system
regulation of the follicular cell functions is weak.
Regulation of the Function of the
Parafollicular Cells
The autonomic nervous
system regulates the function of the parafollicular cells. If the sympathetic
nerve fibers are excited, the parafollicular cells release hormones. If the
parasympathetic nerve fibers are excited, the cells do not release hormones.
The Thyroid Gland Blood Supply
The thyroid gland is
rich with blood and lymphatic capillaries. The capillaries lie closely with
follicular wall.
The prolonged
hyperfunction of the thyroid gland results in the disease called the thyreotoxicosis
characterized by the high metabolism, excessive sweating, abnormal heartbeat,
and exophthalmos.
The prolonged
hypofunction of the children thyroid gland results in the disease called the myxedema
characterized by the body growth delay, the mental development delay, the
decreased metabolism, the rough skin, the tongue volume increase, and the
salivation.
The low function of the
thyroid gland in adult may result in the mental disturbance.
The Thyroid Gland Regeneration
The regeneration of the
thyroid gland is provided by the follicular cell division. The regeneration may
be intrafollicular and extrafollicular.
The intrafollicular
regeneration is
characterized by the formation of the folds consisting of the dividing
follicular cells. The folds are directed towards the follicle lumen. The
follicle becomes star shaped.
The extrafollicular
regeneration is
characterized by the folds directed outwards the follicle. The folds are
separated from the follicle and converted to the small follicles. The cells of
these follicles are multiplied release the secretion into the follicle lumen so
the normal follicle is formed.
The Parathyroid Glands
The parathyroid glands
are called so because they lie closely to the thyroid gland. In common there
are two or four parathyroid glands.
The Development of the Parathyroid
Glands
The parathyroid glands
are derived from the epithelium of the III and IV branchial pouches on the 5th
of week of the embryonic life to form the outgrowths. The outgrowths are
separated from the pouches to form the parenchyma of two or four glands. The
capsule and stroma of the parathyroid glands are derived from the mesenchyme.
The Structure of the Parathyroid
Glands
Every gland is covered by
the connective tissue capsule, from which the septa extend to form the stroma.
Clusters of the parenchymal cells (Fig. 112) called parathyroid cells are
embedded into the stroma. They are round and slightly basophil cells. The cells
are joined by the desmosomes and digiform intercellular junctions. The
parathyroid cells contain well-developed rough ER, Golgi complex and
mitochondria. These cells are divided into 2 types: 1) the chief parathyroid
cells and 2) the oxyphil (acidophil) cells. The latter appear at the age of 6.
Their cytoplasm contains a number of mitochondria therefore these cells are
acidophil. The chief parathyroid cells are divided into the pale principal
cells and dark principal cells.
The parathyroid glands produce the
parathyroid hormone (or parathormone). The osteoclasts have receptors to this
hormone (the osteoclasts are the target cells of parathormone). If the
parathormone level in blood is high, the osteoclasts perceive this hormone. It
activates the osteoclasts therefore they destroy the bone as a result much free
calcium is formed. Calcium enters the blood. Thus, parathyroid hormone tends to
increase level of serum calcium by: 1) the increasing bone resorption through
stimulation of the osteoclast activity, 2) the increasing calcium resorption
from the renal tubules (and inhibition phosphate resorption), and 3) the
enhancing calcium absorption from the gut. The parathormone acts against of the
calcitonin.
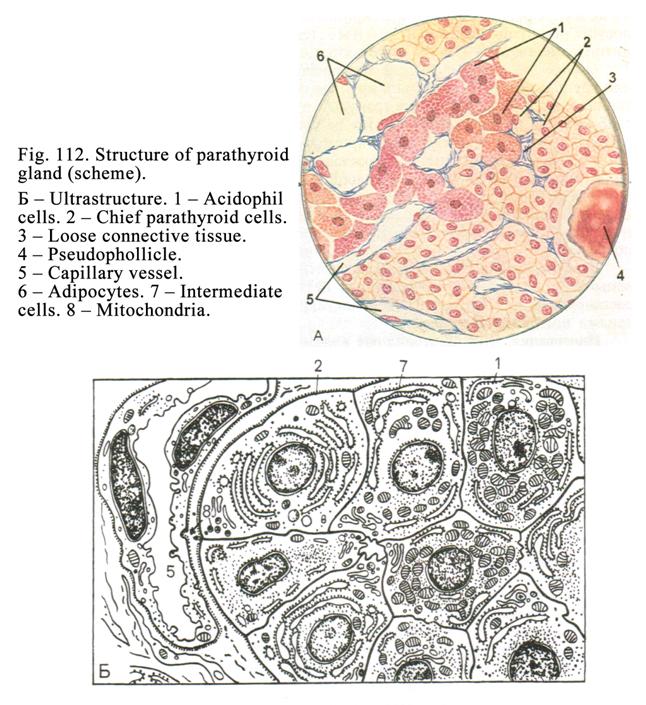
If all parathyroid
glands are removed, convulses begin and patient dies. The cause of convulses is
the decrease of calcium level in the blood and cisterns of the smooth ER of the
myocardium and skeletal muscles.
The Function Regulation of the
Parathyroid Glands
2 factors regulate the function
of the parathyroid glands: 1) the autonomic nervous system and 2) blood calcium
level. If the sympathetic nerve fibers are excited, the parathyroid glands
release the hormone. When the parasympathetic nerve fibers are excited glands
do not release the hormone.
But more effective way
of regulation is blood calcium level. If the level of the blood calcium is
decreased, the parathyroid glands release the hormone more actively. If the
level is high, the gland stops the hormone to release.
The Suprarenal Glands (Adrenal
Glands)
In the human body there
are two suprarenal glands (right and left). Every suprarenal gland actually
consists of 2 glands: 1) the adrenal cortex and 2) the medulla.
The Cortex Development
On the fifth week of the
embryonic life mesoderm visceral cells form two interrenal bodies at the
internal part of the mesentery. These cells are acidophilic. They are the
origin of the fetal adrenal cortex. After the birth the fetal cortex usually
disappears. Sometimes it forms thin layer between the definitive cortex and
medulla. The fetal cortex releases dehydroepiandrosterone (DHA) of the embryo.
The DHA travels to the liver. Here it is converted into a-precursor. The latter is the origin
of chorionic honadotropin synthesized by the placenta.
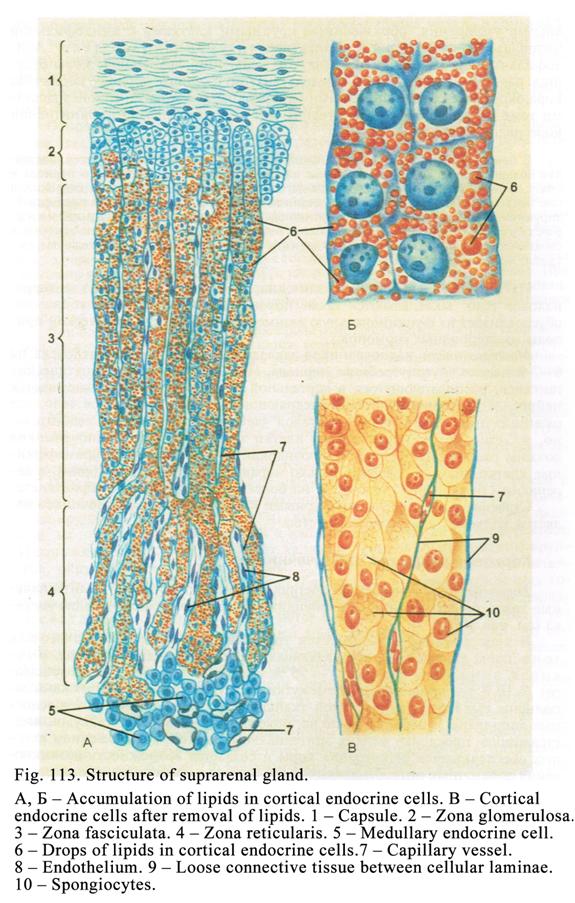
On the tenth week of the
uterine life the surface of the interrenal bodies are covered by other cells of
the visceral mesoderm having basophil cytoplasm. These cells are the origin of
the definitive cortex.
The Medulla Development
The medulla of the adrenal
glands is derived from the nerve crest. The crest cells undergo differentiation
to form the sympathetic nerve blasts. The blasts migrate to the aorta and
accumulate here. Then they migrate into the interrenal bodies to form the
adrenal medulla.
The Suprarenal Gland Structure
The connective tissue
capsule covers the gland. From the capsule septa extend into the gland
substance. The capsule is composed of 2 layers: the internal loose layer and
external dense layer. In the internal layer there are the venous and arterial
plexuses. Deep in the capsule space there is the layer of the stem cells. They
are the origin of the cortex endocrine cells. Deep in this layer there is the
adrenal cortex.
The Cortex Structure (Fig. 113. 2,
3, 4)
The cortex consists of
the cortical cord endocrine cells (Fig. 113). Between the cords thin connective
tissue layers are seen in which fenestrated capillaries are rounded by the
pericapillary spaces. The cortical endocrine cells produce corticosteroids.
Precursors of the corticosteroids are lipids therefore in the cortical
endocrine cells there are incorporations of lipids.
Depend on arrangement
and shape of the epithelial cells in cords there are 3 zones of the adrenal
cortex: 1) zona glomerulosa (its thickness constitutes about 15%), 2) zona
fasciculata (its thickness is about 75%), and 3) zona reticularis (its
thickness is about 15%).
The zona glomerulosa epithelial cells are arranged into
glomerulus-like groups. These cells are often cuboidal or cone shaped. They
contain insignificant amount of lipids. In these cells there are well developed
smooth ER, Golgi complex, and mitochondria containing lamellar cristae. Their
nuclei are round or oval. The zona glomerulosa endocrine cells release
corticosteroid called aldosterone. The hormone stimulates 1) reabsorption of
sodium, chlorine, and carbonates from the renal tubules to the capillaries and
2) enhances inflammation.
Deep to the zona
glomerulosa there is sudanophobic layer consisting of 3-4 rows of small
cuboidal cells. In these cells there is no lipid therefore they are not stained
by
Under the sudanophobic
layer the zona fasciculata is located. It consists of large cuboidal or
columnar cells, forming parallel cords, directed perpendicularly in relation to
the gland surface. The cells contain great amount of lipid. In their cytoplasm
there are well-developed smooth ER, Golgi complex, and mitochondria containing
tubular cristae.
The endocrine cells of
the zona fasciculata are divided into the pale and dark cells (the number of
pale cells is more than dark ones). In the dark cells there is no lipid but
they contain the ribosomes and rough ER. The pale and dark cells belong to the
different phases of the secretory process. The rough ER synthesizes enzymes
taking part in the synthesis of corticosteroids.
The zona fasciculata produces
hormones called glucocorticoids. There are 3 active glucocorticoids: cortisol,
cortisone, and corticosterone (all other glucocorticoids are metabolites). The
most active hormone is cortisol. The actions of glucocorticoids are. 1) They
regulate metabolism of carbohydrates, proteins, and lipids. 2) Glucocorticoids
provide the gluconeogenesis (formation of carbohydrates from proteins and
lipids). 3) They have anti-inflammatory effect. 4) Glucocorticoids destroy
lymphocytes and acidophilic granulocytes if the level of corticoids in blood is
high. 5) These hormones regulate phosphorylation to accumulate energy. 6) They
take part in the stress-reaction.
The stress-reaction
consists of 3 stages: 1) trouble stage (the thread has not been defined yet),
2) resistant stage (the adrenal cortex releases glucocorticoids actively so
lymphocytes and acidophilic granulocytes are destroyed), and 3) the breakdown
stage, which can result in death. The cause of the stress-reaction may be a
disease, the loss of material values, etc.
Corticosteroids are the
nuclear hormones perceived by the nuclear receptors therefore they directly influence
the chromosome genes.
The zona reticularis consists of the epithelial cords,
which interlace to form the reticulum. The cells of this zone are cub, oval, or
conic in shape. Their size is less than those of the zona fasciculata. The cells
contain a little lipid. The zona reticularis cells contain well developed
smooth ER, Golgi complex, and mitochondria. The mitochondria have vesicular
cristae.
The zona reticularis
produces androgens (the male sex hormones), progesterone, and estrogens (the
female sex hormones).
The Adrenal Medulla
The adrenal medulla
(Fig. 113. 5) is located in the gland center. Its stroma is made up of loose
connective tissue. Parenchymal cells of the medulla are lighter than those of
the cortex. The cells are round, oval, or polyhedral. They are called the
medullar endocrine cells. The cells contain well developed Golgi complex, rough
ER, mitochondria, and membrane bound granules (100-500 nm in diameter). In the
granules catecholamines (adrenaline and noradrenaline) are accumulated.
The medullar endocrine
cells are divided into the pale cells, releasing adrenaline, and the dark cells
releasing noradrenaline. These cells are stained by the solution of chromium
salts therefore they are called the chromaffin cells. These cells are also
stained by silver and osmium so they are called argentophil or osmiophil cells
respectively.
Nerve Supply of the Suprarenal
Glands
The efferent
(sympathetic and parasympathetic) nerve fibers are terminated by the motor
terminals on the blood vessel smooth muscle cells of the adrenal cortex. But
these nerve fibers influence the adrenal cortex function is weak.
The sympathetic nerve
fibers innervating the medullar endocrine cells are distinct from that in other
glands because these cells are innervated by the preganglionic nerve fibers
similar to the sympathetic ganglion cells (the medullar endocrine cells are in
fact the sympathetic ganglion cells). When the sympathetic nerve fibers are
excited, the medullar endocrine cells release adrenaline and noradrenaline.
The Adrenal Cortex Function
Regulation
In the regulation of the
adrenal cortex the humoral mechanism takes part. The ACTH stimulates the
hormones synthesis of the zona fasciculata and zona reticularis.
The stimulation
aldosterone synthesis by the zona glomerulosa differs than that of the zona
fasciculate and zona reticularis. At first the ACTH stimulate the
corticosterone synthesis. Then rennin (the kidney endocrine cells produce the
rennin) influences the corticosterone to produce aldosterone. In addition the
cerebral epiphysis hormone stimulates the aldosterone synthesis. Natriuretic
hormone, produced by the cardiac myocytes, inhibits the aldosterone synthesis.
Blood Supply of the Suprarenal
Glands
More than 30 arteries
enter the suprarenal gland. These arteries form the arterial plexus in the
gland capsule internal layer. From this plexus a number of the capillaries
extend to the gland parenchyma. The capillaries round epithelial cords and
reach the medulla. Here they join
the sinusoid of the medulla (medullar venous plexus). The sinusoids join larger
vessels to form 3-4 large sinusoids. The latters join together to form the
central vein entering the inferior vena cava. In the wall of the central vein
and large sinusoids there are the smooth muscle sphincters regulating blood
flowing.
The Adrenal Medulla Blood Supply
From the capsular
arterial plexus arterioles pass through the cortex to the medulla. Here they
ramify to form capillaries applying to the basal part of the medullar endocrine
cells. Then the capillaries join sinusoids. The apical part of the medullar
endocrine cells applies to the sinusoids. From the capillary blood various
substances (water, amino acids, ions, etc.) are passed in the basal part of the
medullar endocrine cells. From the apical part of these cells catecholamines
enter the sinusoids.
The venous blood can
drain from the medulla into the inferior vena cava and into the portal vein of
the liver (the second pathway). Between the sinusoids of the medulla and of the
adrenal capsule venous plexus there are some anastomoses. When sphincters in
the wall of the large sinusoids and central vein close, the venous blood,
containing catecholamines and corticosteroids, flows to the capsular venous
plexus. From the plexus some veins arise falling into the spleen vein, inferior
mesenteric vein, and other veins, which enter the portal vein.
The venous blood only
travels from the medulla to the portal vein in the extraordinary conditions
(insulin shock, heart shock, etc.). At the same time adrenaline is used for the
liver glycogen splitting to form glucose entering the blood. At the same time
the excess of corticosteroids is destroyed.
Age Dependent Changes of the Adrenal
Glands
The complete development
of the suprarenal glands is provided in 20-25 years. At this time the zona
glomerulosa constitutes 1 part, zona fasciculata constitutes 6 parts, and zona
reticularis constitutes 3 parts. In elderly the zona glomerulosa becomes thin
zona reticularis also becomes thin but less than zona glomerulosa. The lipids
amount in the cells is less. It leads to the decrease of the corticosteroid
synthesis.
The gland medulla is not
essentially changed but in the very old age sclerosis of the blood vessels is
seen. It leads to the medulla atrophy.
Diffuse Endocrine System
The single endocrine
cells scattered in the various parts of the body represent this system. The
most number of these cells have neurogenous origin. These cells constitute the
neuroendocrine or APUD (Amino Precursor Uptake and Decarboxylation) system.
The neuroendocrine cells
are located in the epithelium of the gut, in the epithelium of the respiratory
pathway, in the epithelium of the urinary pathway, and in some endocrine organs
(parafollicular cells of the thyroid gland, cells of the medulla of the adrenal
glands, and cells of the epiphysis cerebry). The English scientist Pierce was
the first to describe this system.
The neuroendocrine cells
have amount of properties. They: 1) contain nervous amines and small peptide
hormones, 2) contain dense secretory granules, 3) posses ability to be stained
by metal salts, and 4) posses ability to uptake and decarboxylation precursors
of amines.
Origins of the Neuroendocrine Cells
Development
Origins of the
neuroendocrine cells are 1) the nervous ectoderm (the hypothalamus, epiphysis,
the medulla of the adrenal glands, and neuroendocrine cells of the central and
peripheral nervous system), 2) the dermal ectoderm (adenohypophysis, and
Merkels cells), 3) the endoderm (endocrine cells of the gut), 4) the mesoderm
(cardiac myocytes releasing natriuretic hormone), and 5) the mesenchyme (mast
cells).
In the body there are
cells, which are not developed from the nervous origin (interstitial cells of the
male testis, and follicular cells of the female ovary). They are derived from
the visceral mesoderm cells.
The solitary endocrine
cell possesses the ability to the paracrine and distant action i.e., the
hormones released by the cell influence the adjacent cell (it is the paracrine
action) on the other hand the hormone released by the cell enters the blood and
influence the distant cell (it is the distant action).
CHAPTER
17
HEMOPOIETIC
ORGANS & BONE MARROW & HEMOPOIESIS
The hemopoietic organs are divided into the central and peripheral organs. The central organs are the bone marrow, thymus, and bursa of Fabricus. In birds there is bursa but in man it is not observed. In man there is structure, which is similar to the bursa. But nobody knows where the structure is located.
The peripheral organs are the
spleen, lymphatic nodes, and mucosa associated lymphoid tissue (the gut, the
airways of the respiratory organs, the urinary pathways, and others).
All hemopoietic organs (except
the thymus) are derived from the mesenchyme. The thymus is derived from the
bronchial pouches (III pair).
The structure of the
hemopoietic organs is identical for all of them. They consist of the
hemopoietic cells and stroma. The stroma of all the hemopoietic organs (except
the thymus) is represented by the reticular tissue. The stroma of the thymus is
the epithelium.
MIELOID ORGANS
The myeloid hemopoietic organs
are represented by the myeloid tissue. The myeloid organ is the bone marrow, in
which all cellular or formed elements of blood are developed (erythrocytes,
leucocytes, and platelets).
The
Lymphoid Organs
The lymphoid organs are made
up of the lymphoid tissue. The lymphoid tissue is the cluster of lymphocytes
and other cells. The lymphoid organs are the spleen, thymus, lymphatic nodes,
and lymph nodules.
The hemopoietic organs provide
1) blood formation, 2) blood break down, 3) defence function, and 4)
accumulative function.
The Bood Formation Regulation
The central nervous system,
endocrine system, immune system, surrounding cells, and intra tissue mechanism
regulate the blood formation.
The
surrounding cells are stroma
cells and macrophages providing the phagocytosis and releasing hemopoietins,
which stimulate the hemopoietic cells maturation.
The intra tissue
mechanism is chalons released by mature
neutrophilic granulocytes. The chalons inhibit development of young cells.
After the maturation the maturating hemopoietic cells enter the blood stream.
Some of blood cells (erythrocytes, platelets) circulate in the blood till they
die while other cells (leucocytes) circulate in the blood for some hours then
they enter the tissues and maintain their function there.
The Blood Formation Stages
The primary hemopoiesis provides in the yolk sac at the embryonic life. After it the hemopoiesis begins in the liver and spleen. In the liver the hemopoiesis is maintained in the embryo till the birth time. In the spleen the blood formation is increased by the birth and prolonged after that. In the bone marrow hemopoiesis take place before the birth and after it.
Hemopoiesis
in the Bone Marrow
The bone marrow (Fig. 114) is the central hemopoietic organ containing stem cells. From the stem cells erythrocytes, granulocytes (neutrophilic, eosinophil, and basophil), monocytes, B-lymphocytes, precursors of T-lymphocytes, and platelets are derived. In the bone marrow the antigen-nondependent differentiation on the B-lymphocytes occurs. The surrounding cells of the bone marrow are the reticular cells, macrophages, osteogenic cells, and adipose cells that are seldom divided.
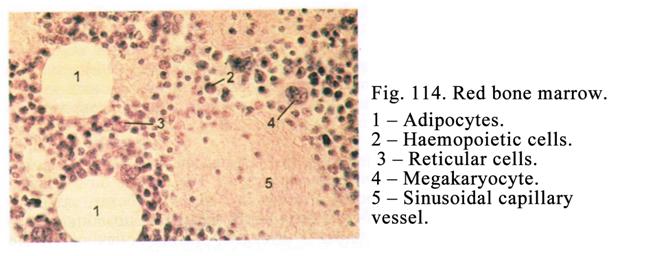
The stroma cells of the bone marrow are derived from the mesenchyme. The blood cells are derived from the stem cells, derived from the mesenchyme. The stem cells are seldom divided.
The primary bone marrow forms in the clavicle on the second month of the embryonic life. On the third month the bone marrow forms in the membranous bone at last it forms in the long bone diaphysis. On the fifth-sixth months in the bone diaphysis the bone marrow cavity is formed. And the bone marrow becomes the major hemopoietic organ.
In the children at the age of 12-18 the bone marrow is localized in the bone diaphysis later it is replaced by the yellow bone marrow. Thereafter the red bone marrow is localized only in the bone epiphysis and the membranous bone.
In common the red bone marrow mass constitutes 4-5% of the human body. The bone marrow colour is red and semifluid. In the bone marrow cavity hemopoiesis is provided in its periphery because a number of stem cells are the greatest there.
The hemopoietic cells form groups located in the bone marrow stroma loops. That is, erythroblasts are arranged around macrophage because it gives them iron molecules necessary to form hemoglobin. On maturation erythroblasts are converted to erythrocytes entering the blood stream through the sinusoid wall. The small amount of erythrocytes is accumulated in the bone marrow. Reticulocytes (young erythrocytes) are matured in the bone marrow or peripheral blood vessels per 1-2 days.
Granulocytes form groups as well. The matured granulocytes enter the blood stream but the most part of them is accumulated in the bone marrow. They may be thrown into the blood stream at any moment. It can explain the rapid increase of granulocytes in the blood stream in diseases.
Agranular leucocytes are arranged around the blood vessels as belts. Megakaryocytes are localized near the sinusoid vessels. The cell process enters the sinusoid lumen. From the process small particles (platelets) are separated and carried by the blood flow.
Commonly in to the blood stream only matured blood cells can enter. As for unmatured blood cells they can leave the bone marrow only in case of disease. It is so unmatured blood cells seen to be larger than the matured cells. For example, the erythroblast is 18 mm in diameter while the matured erythrocyte is only of 8 mm in diameter.
The Yellow Bone Marrow
The yellow bone marrow appears in the long bone diaphysis after the puberty. It contains a great amount of lipid tissue, consisted of the adipose cells, having yellow pigments, called the lipochromes. In the normally the yellow bone marrow does not provide the hemopoietic function, but in case of disease or loss of blood the stem cells enter the yellow bone marrow to start hemopoiesis.
The Bone Marrow Supply
From the periosteum the artery enters the bone marrow. In the bone marrow cavity center the artery is divided (bifurcate) into the ascending and descending branches. From these branches the thin (2-4 mm in diameter) capillary vessels arise. Only blood plasma can passes through the capillary vessels. When the capillary vessels approach the marrow cavity wall, their diameter becomes large (20-30 mm in diameter) i.e., they are converted into the sinusoids. Through the sinusoid walls the matured blood cells enter the sinusoid lumen from the bone marrow. The sinusoid vessels run towards the bone marrow cavity center and fall into the vein, which is located near the arterial branches. The vein diameter is similar to that of the artery therefore the pressure in the sinusoids is high and the sinusoid walls are not collapsed.
Thus, the blood, entering the bone marrow, brings it oxygen and nutrients and takes in the blood elements (cells) as well.
In addition the blood enters the bone marrow from the arteries passing through the osteon canals and perforating canals. This blood contains salts influencing the blood formation in the bone marrow.
The
Bone Marrow Regeneration
When the part of the bone marrow is removed, its reticular stroma is replaced due to division of the persisting reticular stroma cells (undifferentiated reticular cells). The bone marrow hemopoietic cells are replaced due to entering new stem cells.
Age Dependent Changes of the Bone
Marrow
In the bone marrow of the newborn most of the cells are erythroblasts. In the puberty the bone marrow is similar to that of the adult. In the elderly the bone marrow becomes gelatinous.
Blood
Formation in the Bone Marrow
The embryonic blood formation in the bone marrow begins on 11-12th week the post embryo one is prolonged.
According to recent research all the blood cells are derived from one stem cell. These researches correspond to the monophyletic theory suggested by A.A. Maksimov. He believed that structure of the cell, which is origin for all the formed blood elements morphologically similar to the lymphocyte. And there were polyphyletic theories. According to one of them all the blood cells are derived from 3 initial cells according to other theory all the blood cells are derived from 5 cells. At present these theories are not confirmed.
Blood formation in
the bone marrow is called the hemopoiesis. In the blood smear the stem cell and
lymphocyte is impossible to identify, they are similar. The stem cell may be
distinguished from the small lymphocyte if it (stem cell) is sowed in the
spleen of the mouse, which undergoes the lethal dose of radiation. In this case
the stem cell gives the colony-forming unit while lymphocyte does not do that.
Using this method the scientists reveal that 100000 cells of the bone marrow
include 50 stem cells. In the spleen there are about 3 stem cells and 1-2 stem
cells in the peripheral blood.
The
Hemopoietic Cell Classes
The hemopoietic cells are divided into 6 classes. The first class is the stem cells, the second class is the semi-stem cells, the third class is the monopotent precursors, the fourth class cells is the blasts, the fifth class cells is the differentiating cells, and the sixth class is differentiated blood cells (Scheme 1).
The First Class Cell Properties
These cells possess following properties: 1) they are similar to the lymphocytes, 2) they divide rarely, 3) they are totipotent, 4) they are not determined, 5) they are self renewing (i.e. they can divide to form new cells of their own type), and 6) they form colony forming units.
The Second Class Cell Properties
These cells possess properties as follows: 1) they are similar to lymphocytes, 2) they divide rarely, 3) they are pleuripotent, 4) they are partly determined, and 5) they form colony-forming units.
The Third Class Cell Properties
These cells possess properties as follows: 1) they are similar to lymphocytes, 2) they divide rarely, 3) they are mono-potent, 4) they are determined, and 5) they form colony-forming units.
The Fourth Class Cell Properties
These cells called blasts contain round nucleus with loose chromatin and nucleoli. Their cytoplasm is basophilic their diameter is 18-20 mm. From each blast only one type of cell is derived. The ffifth class cells are called the differentiating cells.
The sixth class cells are the mature cells.
Development of the Neutrophilic
Granulocytes to the Myeloblast Stage
The stem cell is
the first then semi-stem sell called the pleuripotent hemal cell, then CFU-GM
(colony forming unit granulocytes and monocytes), then the neutrophilic
myeloblast (Scheme 1).
Development of the Eosinophil Granulocytes to the Myeloblast Stage
The stem cell is the first the second is the pleuripotent hemal stem cell the third is CFU-E (colony forming unit of eosinophil granulocytes) the fourth cell is eosinophil myeloblast.
Development of the Basophil Granulocytes
to the Myeloblast
The first is the stem cell the second is the pleuripotent hemal stem cell the third is CFU-B (colony forming unit basophil granulocytes) the fourth is the basophil myeloblast.
Three types of myeloblasts give rise to the promyelocytes (neutrophilic, eosinophil, and basophil). The promyelocytes give rise to the myelocytes (neutrophilic, eosinophil, and basophil). The myelocytes give rise to the metamyelocytes (neutrophilic, eosinophil, and basophil). The metamyelocytes give rise to the rod-nuclear neutrophilic and eosinophil granulocytes). The rod-nuclear neutrophilic granulocyte gives rise to the segmento-nuclear granulocyte the rod-nuclear eosinophil granulocyte gives rise to the eosinophil segmento-nuclear granulocyte. The basophil metamyelocite gives raise the matured basophil granulocyte (the rod-nuclear basophil granulocytes are absent).
The Myeloblast Structure
The myeloblasts (the fourth class cells) are similar to all the blasts. They are round cells their diameter is 18-20 mm they contain round or oval nuclei containing euchromatin and nucleoli. The cell cytoplasm contains number of ribosomes therefore it is basophil. The neutrophilic, basophil, and eosinophil myeloblasts do not distinguish from each other. (It is, however possible that neutrophilic, eosinophil and basophil granulocytes arise from common cell, called the myeloblast).
The Promielocyte Structure
Neutrophilic, eosinophil, and basophil promyelocytes do not also distinguish from each other. They are round, contain round nuclei with nucleoli, their cytoplasm is basophilic. The cell cytoplasm contains well-developed cytocenter, Golgi complex, and lysosomes (or primary granules).
The Myelocyte Structure
The myelocytes (the fifth class cells) are oval, contain oval nuclei without nucleoli, their diameter is 12-18 mm. In their cytoplasm there are common granules and specific granules. In the neutrophilic myelocytes the specific granules are stained by both basic and acid dyes. The specific granules of the eosinophil myelocytes are stained by the acid dyes. In the basophilic myelocytes these granules are stained by the basic dyes. All myelocyte types are actively divided. Neutrophilic, eosinophil, and basophilic myelocytes can be recognized. The myelocytes, particularly neutrophilic, provide the phagocytosis function.
The Metamyelocyte Structure
The metamyelocytes
(the fifth class cells) are formed due to division and differentiation of the
myelocytes. They lose the ability to mitosis, their nuclei are bean shaped, and
their cytoplasm contains more specific granules than myelocytes. If the
neutrophilic metamyelocyte enter the blood stream, it is called the young
neutrophilic granulocyte. The
metamyelocytes are able to move.
Rod Nuclear Granulocytes
The young neutrophilic and eosinophil granulocytes have rod-like nuclei. But the rods are curved therefore the nuclei become C-shaped or S-shaped. They belong to the fifth class cells.
Segmento-nuclear Neutrophilic and
Eosinophil Granulocytes
These cells belong to the sixth class cells. The eosinophil granulocyte nuclei consist of 2 or 3 segments connected by the fine threads, nuclei of the neutrophilic granulocytes consist of 2-7 segments.
The maturated basophilic granulocytes usually have oval nucleus.
In normally the constant level of the granulocytes in the blood is provided by the division of the myelocytes. In case of profuse blood lost the more young cells than myelocytes (also stem cells) are divided.
In the process of the granulocytopoiesis the following changes are seen: 1) after the myeloblast stage the size of the cells is decreased, 2) the shape and structure of the cell nuclei are altered (in the myeloblast the nucleus is round, in the matured neutrophilic granulocyte it is segmented), 3) in the myelocytes and following cells the specific granules appear, and 4) the ability to cell mitosis is lost (the metamyelocytes are not able to divide).
The Erythrocytopoiesis (Scheme 1)
The first is the totipotent stem cell, from which the chain of the hemopoietic cells follow: 1) totipotent stem cell, 2) CFU-GEMM (colony forming unit of granulocytes, erythrocytes, monocytes, and megakaryocytes), 3) BFU-E (burst forming unit of erythrocytes), 4) CFU-E (colony forming unit of erythrocytes), 5) erythroblast, 6) proerythroblast, 7) basophilic erythroblast, 8) polychromatophilic erythroblast, 9) acidophilic erythroblast, 10) reticulocytes, and 11) erythrocyte.
The Burst Forming Unit (BFU-E)
It is the third class cell. It is less differentiated than CFU-E. The BFU-E multiplies very rapidly (in 10 days it provides 12 divisions). This explosive growth gives this cell the name burst-forming unit. Erythropoietin weakly stimulates this cell while interleukin 3 (IL-3) stimulates it strongly. IL-3 is produced by macrophages and T-lymphocytes. The BFU-E cells are in small amount in the bone marrow and blood.
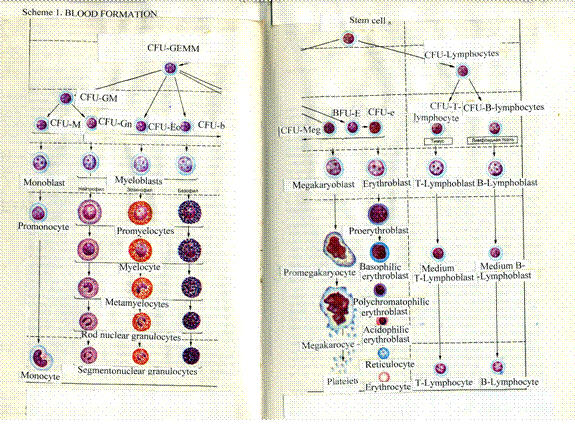
The CFU-E Cells
They are main origins, from which erythrocytes are formed. The CFU-E cells arise from BFU-E cells. The erythropoietin influence the CFU-E cells actively so they are converted into erythroblast (the fourth class cells).
The Erythroblasts (The Fourth Class Cells)
The erythroblasts are similar to the other blasts. They are round, their diameter is about 20 mm, and their round nuclei contain a euchromatin and nucleoli. The erythroblast cytoplasm is slightly basophilic.
Proerythroblasts (The Fifth Class Cells)
They are formed due to the multiplication and differentiation of the erythroblasts. The proerythroblasts are 14-18 mm in diameter. They contain round nucleus with euchromatin and nucleoli. Their basophilic cytoplasm contains ribosomes, polyribosomes, Golgi complex, and rough ER.
Basophilic Erythroblasts (The Fifth Class
Cells)
Precursor cells of the basophilic erythroblasts are proerythroblasts. The erythroblasts are 13-16 mm in diameter. These cells contain round nuclei containing rough clusters of chromatin. The cell cytoplasm is deep basophilic because it contains a large amount of ribosomes, where hemoglobin is synthesized.
Polychromatophilic Erythroblasts (The
Fifth Class Cells)
They are formed from the basophilic erythroblasts. These cells are round their diameter is 10-12 mm. Their round nuclei contain a large amount of heterochromatin. The cell ribosomes synthesize hemoglobin. As the hemoglobin is formed some areas of cytoplasm become eosinophil while others remain basophilic. Therefore they are called polychromatophilic. These cells are rapidly divided.
Acidophilic Erythroblasts (The Fifth Cass
Cells)
These cells are derived from the polychromatophilic erythroblasts. The acidophilic erythroblasts are 8-10 mm in diameter. Their round small nuclei are hyper-chromatic as they undergo pycnosis. The cell cytoplasm contains a large amount of hemoglobin therefore the entire cytoplasm is stained by the acid dyes. These erythroblasts lose the ability to divide.
Reticulocytes (The Sixth Class Cells)
They are formed from the acidophilic erythroblasts losing their nuclei. The reticulocyte cell cytoplasm contains remnants of ribosomes and mitochondria forming basophilic reticulum. Therefore they are called reticulocytes. The reticulocytes are matured in 1-2 days. They undergo maturation in the bone marrow sinusoids or peripheral blood vessels.
Erythrocytes (The Sixth Class Cells)
They are formed from the reticulocytes their diameter is 7-8 mm.
In normally the constant level of erythrocytes is due to the division of the polychromatophilic erythroblasts. In case of profuse blood lost the greater amount of younger cells (basophilic erythroblasts, proerythroblasts, erythroblasts, mono-potent precursors and others cells) are rapidly divided.
The Alteration Watched in the Process of
the Erythrocytopoiesis
Processes taking place simultaneously are: 1) the cell size is decreasing, 2) hemoglobin is accumulated in the cell cytoplasm of the developing cells, 3) the nucleus structure undergoes alteration and the cell leaves the nucleus, and 4) the ability to divide is lost (the acidophilic erythroblast cannot divide).
The Megakaryocytopoiesis
From the totipotent stem cell the chain of the hemopoietic cells follows: 1) totipotent stem cell, 2) CFU-GEMM, 3) CFU-MKC (CFU-mono-potent precursor of megakaryocytes), 4) megakaryoblast, 5) promegakaryocyte, 6) megakaryocyte, and 7) thrombocyte (platelet) (Schem 1).
The Megakaryoblast
The cell is 15-25 mm in diameter its nucleus has invagination. The megakaryoblast can divide.
The Promegakaryocyte
The promegakaryocyte is formed from the megakaryoblast. The cell loses the ability to divide while get the ability to endomitosis (nucleus is dividing without cytoplasm division) so its nucleus becomes multi-lobed and polyploidy (4n-8n). The size of the nucleus and cytoplasm are increased. Azurophilic granules are accumulated in the cell cytoplasm.
Megakaryocytes
The promegakaryocytes become large to form megakaryocytes. They are large cells (50-100 mm in diameter). The megakaryocytes are divided into 1) the reserve megakaryocytes and functional (matured) megakaryocytes. The reserve megakaryocytes are large polyploidy cells (50-70 mm in diameter and 16-32n). They do not produce platelets. The matured megakaryocytes are 50-100 mm in diameter and 32-64n. They produce the platelets. In their cytoplasm the chains, consisted of vesicles, are formed. These chains are boundaries dividing the cell cytoplasm into the small parts (platelets). These parts are separated from the cytoplasm to form platelets. Thereafter the cytoplasm is sharply decreased as a result the cell becomes small. This cell is called the residual megakaryocyte, which is engulfed by the macrophages or is destroyed.
The Monocytopoiesis
The first cell is 1) totipotent stem cell, 2) the second cell is CFU-GEMM, 3) the third cell is CFU-GM, 4) the fourth cell is CFU-M, 5) the fifth cell is monoblast, 6) the sixth cell is promonocyte, and 7) the seventh cell is monocytes (Scheme 1). From the bone marrow monocytes enter the blood stream, where they live 2-4 dyes. Then monocytes migrate into the connective tissue and are converted into the macrophages.
CHAPTER
18
LYMPHATIC
ORGANS & LYMPHOCYTOPOIESIS
The lymphatic organs are the thymus, spleen, lymphatic nodes, and lymph nodules.
The Thymus
The thymus is
located behind the manubrium sternum. Its weight is 30-
The Thymus Development
The thymus is derived from the epithelium of the pharynx on the 4-5th weeks of the embryo to form 2 bulges. The bulges grow towards the caudal part of the body. Then the distal parts of the bulges are joined to form the epithelial stroma of the thymus bud. Around the stroma the capsule of the thymus is formed from the surrounding mesenchyme. The septa, arising from the capsule, extend into the stroma. These septa are accompanied by the blood vessels. The septa passing from the capsule divide the stroma into the large amount of lobules. In the peripheral part of each lobule the cortex is formed while in the central part the lobule medulla is formed. Some epithelial cells of the medulla undergo keratosis to form thymic corpuscles. The hemopoiesis in the thymus begins on the 8.5-10 the week of the embryo.
The Thymus Structure
The connective tissue capsule covers the thymus. From the capsule the septa pass inwards the thymus. The septa divide the thymus into some lobules. Every lobule is composed of the cortex, located in its peripheral part, and medulla located in the lobule center. The lobule stroma is the epithelium forming the epithelial reticulum because every epithelial cell has processes joining those of the neighboring cells. The stroma epithelial cells lie on the basal membrane applying to the capsule and septa. Basal membrane (in the center of the medulla) undergoes keratosis so the thymic corpuscles are formed (Fig. 115).
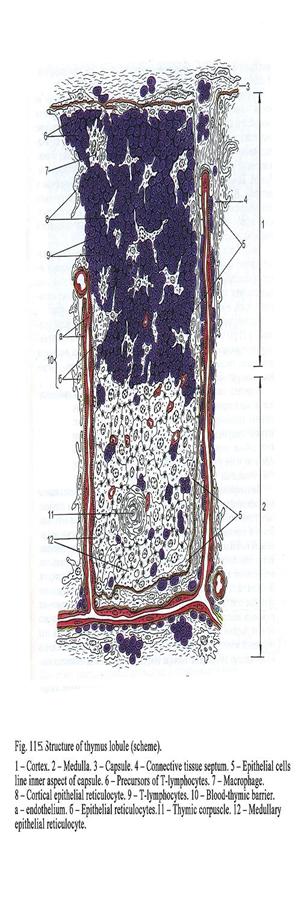
The Lobule Cortex
The cortex is dark colour because it includes a number of lymphocytes. The blood carries the precursors of T-lymphocytes into the cortex from the bone marrow. Here the hormone thymosin and stroma cells influence the precursors as a result they are converted into lymphoblasts the latter undergo multiplication and differentiation non-depending on antigen i.e., differentiation without antigens (antigens are few). Why are not antigens there? They are absent because the cortex capillary vessels are surrounded by the blood-thymus barrier. The barrier is composed 5 components: 1) capillary endothelial cells, 2) the basal membrane of the capillary, 3) pericapillary space including fluid contained macrophages and lymphocytes, 4) the basal membrane of the epithelial stroma, and 5) the epithelial stroma. The barrier prevents pass way of antigens into the cortex.
If the barrier is damaged, it is strengthened by the neutrophilic granulocytes, providing phagocytosis of the antigens, plasmocytes, containing antibodies, and mast cells regulating the permeability of the blood capillaries. If the mast cells release heparin, the permeability of the blood capillary wall becomes low. If they release histamine, its permeability is high.
So the non-dependent on antigen differentiation T-lymphocyte precursors are converted into T-lymphocytes having receptors to antigens. These T-lymphocytes are divided into T-helpers, T-suppressors, and T-killers.
Some T-lymphocytes have receptors to the host. They can destroy the cells of the host. These T-lymphocytes are engulfed by the macrophages.
After the non-dependent on antigen differentiation T-lymphocytes enter the blood stream, which carry them to the peripheral lymphatic organs (lymph nodes, spleen etc.). Here the lymphocytes enter antigen-dependent or T-cells zones and undergo antigen-dependent differentiation.
The Lobule Medulla
The medulla is pale because its stroma contains fewer amounts of the lymphocytes than that of the cortex.
The kind of receptors of these lymphocytes distinguishes from that of the cortex lymphocytes. The medullar lymphocytes can enter the blood stream then come back. It is possible because there is no blood thymus barrier in the medulla. In the medulla center the thymic corpuscles are seen. Each corpuscle has the central core formed by the epithelial cells, which undergo keratosis. Around the core there is the wall formed by concentrically arranged epithelial cells.
The Thymic Lobule Blood Supply
Arteries, entering the thymus, are divided into the interlobular arteries. From every interlobular artery 2 intralobular arteries arise. One artery enters the lobular cortex near the lobular medulla boundary to form the arc. From the arc towards the capsule or trabeculae (septa) the capillaries, surrounded by the blood thymus barrier, arise. These capillaries enter the subcapsular vein. This vein leaves the lobule and enters interlobular vein.
The second intralobular artery enters the lobule medulla. The artery branches to form the medulla capillary network without blood thymic barrier. The capillaries drain into the medullar vein. Llatter drain into the intralobular vein. Thus, the inflow and outflow of the blood into the cortex and medulla are provided by various blood vessels (Fig. 116).
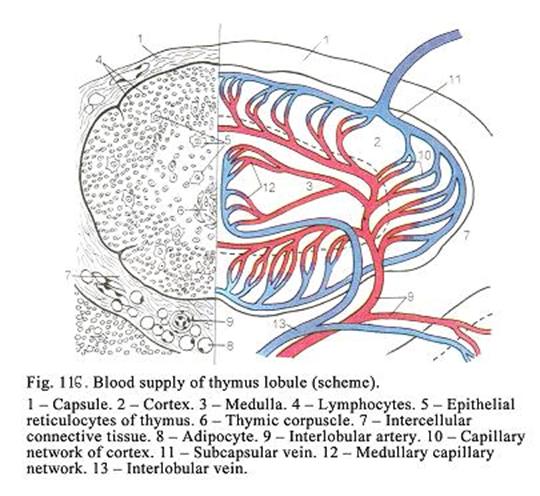
Age Dependent Involution of the Thymus
The thymus is fully developed by 3 years. Till 20 years the thymus is constant. Then it undergoes age involution. Simultaneously the connective tissue of the capsule and trabeculae grow. The adipose tissue grows as well while lymphocytes disappear from the cortex and medulla as a result the thymus tissues are replaced by fats. In this case the precursors of the T-lymphocytes undergo antigen-dependent differentiation in the skin epidermis. If the age-dependent involution does not happen, the person loses ability to the disease resistance. Such condition is observed when the adrenal cortex is inactivated.
The Thymus Temporary Involution
The involution happens when the disease, stress or shock occur. At the same time the adrenal cortex releases more corticosteroids. It leads to break down of the lymphocytes in the cortex and medulla of the thymic lobules so the cortex becomes pale as the medulla. When the process of disease is terminated, the temporary involution is terminated as well.
The Thymus Function
It provides 2 functions: 1) the hemopoietic function (it provides antigen-independent differentiation of T-lymphocytes) and 2) release secretions (it releases thymosin, stimulating the peripheral lymphatic organs development and function, calcitonin-like factor, decreasing the blood calcium level, and insulin-like factor). If the thymus is removed, the development of the peripheral lymphatic organs is disturbed.
Lymphatic Nodes
The lymphatic nodes along with lymphatic vessels constitute the lymphatic system.
Development of the Lymphatic Nodes
The lymphatic nodes are derived from the mesenchyme cell clusters along the blood and lymphatic vessels on the 8-10th week of the embryonic life. From the mesenchyme around the node bud the capsule of the node is formed. Between the capsule and node bud the subcapsular sinus is formed. From the subcapsular sinus the cortical sinuses arise. The cortical sinuses are located between the lymph follicles and trabeculae. From the cortical sinuses the medullar sinuses arise, lodging between the lymph cords and trabeculae. The trabeculae, arising from the capsule, extend into the lymphatic node substance dividing it into small lobules. The mesenchymal cells of the lymphatic node bud are converted into the reticular stroma of the node. The stem cells enter the reticular stroma. Thereafter the hemopoiesis, lasting for some weeks, begins. On the 16th week B-lymphocytes enter the lymphatic node periphery at last T-lymphocytes pass between the center and periphery of node. Then lymphocytopoiesis begins lasting before birth and after birth. On the 20th week the lymphatic node bud assumes the features of the definitive lymphatic node.
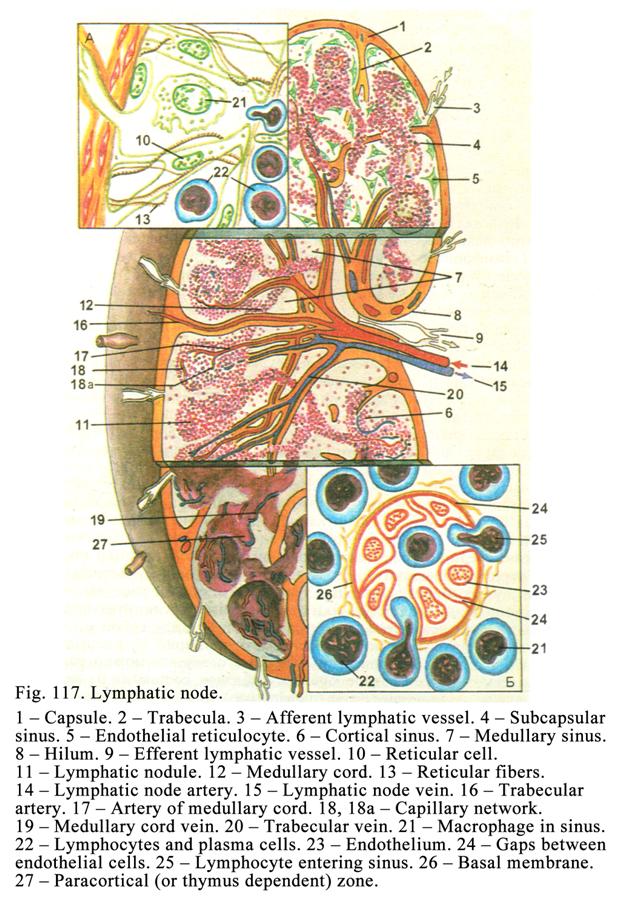
The Lymphatic Node Structure
The lymphatic nodes
(Fig. 117) locate near the blood and lymphatic vessels. They are oval or bean-like.
The lymphatic node is covered by the connective tissue capsule, from which the
trabeculae extend into the node substance. The capsule and trabeculae consist
of collagen and elastic fibers. The smooth muscle cells are present as well. On
the concavity surface of the node there is hilus, through which the artery and
nerve enter and vein along with the efferent lymph vessel leave the node. The
afferent lymph vessels enter its convex surface. The stroma of the lymphatic
node is the reticular tissue consisting of the reticular cells and reticular
fibers. The lymphatic nodes are 0.5-
The Cortex
The cortex is made
up of lymph nodules (they are 0.5-
The free macrophages of the lymph nodules provide engulfing function i.e., they engulf antigens and present them to lymphocytes (antigen presenting function). These antigens stimulate the lymphocytes to the antigen dependent differentiation.
Dendritic cells of the lymph nodues are macrophages lost the ability to phagocytosis. These cells have processes, slightly developed organelles, and slightly stained cytoplasm. Their cell membrane bears receptors to immunoglobulins. These receptors perceive immunoglobulins. The free ends of immunoglobulins receive antigens. These antigens along with antigens of free macrophages and along with T-lymphocytes (helper T-cells) stimulate B-lymphocytes for multiplication, differentiation, and high activity (producing antibodies).
B-lymphocytes enter the lymph nodule (cortex) with the blood stream from the bone marrow. In the lymph nodules antigens of free macrophages, dendritic cells, and T-helper lymphokines influence the pass way of B-lymphocytes so the latter grow to form lymphoblasts (plasmoblasts), undergo multiplication and antigen dependent differentiation. Due to the differentiation B-lymphocytes are converted into the plasmocytes and memory cells. The plasmocytes produce antibodies against antigen, which has influenced the B-lymphocyte. The memory cells remember the antigen influencing B-lymphocyte. Plasmocytes and memory cells enter the blood stream, leave the latter and enter connective tissue where they perform their functions. Plasmocytes synthesize antibodies breaking down their antigen (which has influenced B-lymphocyte). When the memory cell meets its antigen (influencing B-lymphocyte), it multiplies and is converted into the active cells (plasmocytes) releasing the anti-bodies against the antigen and destroying it. Thus, the lymph nodule is B-lymphocytes zona.
Stages of the Lymph Nodule Development
There are 4 stages. When antigen enters the lymph nodule, the first stage begins. At the same time the germinal (pale) center is formed but it is small. Here the lymphoblasts are divided by mitosis. At the second stage the germinal center becomes wider. Here about 10 cell divisions are seen. At the third stage around the germinal center the corona (or mantle), consisting of small lymphocytes, is seen. At the same time the amount of dividing lymphoblasts decreases. At the fourth stage solitary dividing cells are seen. Around the narrow germinal center the bright corona, consisting of memory cells, is seen. The entire cycle lasts for 2-3 days. In one week after the cycle the medullar cords become wider, in the lymphatic sinuses a number of lymphocytes and plasmocytes are seen. If the antigen does not enter the body, there is no germinal center in the lymph node.
The Paracortical Zone
The paracortical zone is located between lymph nodules and medullar cords. In the paracortical zone there are dendritic antigen presenting cells, T-lymphocytes, and T-lymphoblasts. The dendritic antigen presenting cells is macrophages losting their ability to phagocytosis. Their organelles are not prominent their cytoplasm is slightly acidophilic. These cells produce glycoproteins stimulating T-lymphocyte differentiation. The dendritic antigen presenting cell surface bears receptors, which hold antigen, taking part in T-lymphocyte differentiation. In the paracortical zone the interaction between the immune cells (lymphocytes and macrophages) is provided. If the thymus is removed, the paracortical zone is poorly developed. Thus, the paracortical zone is thymus dependent zone.
The T-lymphocytes leaving the thymus enter the paracortical zone from the blood stream. Here the antigens of the dendritic antigen presenting cells influence these T-lymphocytes so they are converted into the effector cells (helper T-cells, suppressor T-cells, cytotoxic T-cells, and memory cells).
Delayed type hypersensitivity related T-cells synthesize and release lymphokines when they come in contact with antigens. The lymphokines attach macrophages and neutrophilic granulocytes into this area. These cells destroy antigens.
Helper T-cells stimulate production of antibodies by B-lymphocytes and plasmocytes (stimulate their functions).
Ssuppressor T-cells inhibit the activity of B-lymphocytes and plasmocytes and their antibodies production.
The helper T-cells and suppressor T-cells interact with antigens, which include major histocompatibility antigen complex of II class (class II MHC). They are bacteria, viruses, fungi, at etc. The helper T-cells and suppressor T-cells regulate the humoral immunity.
The cytotoxic T-cells (killers) provide cellular immunity. They attack and destroy other (foreign) cells (by release of special proteins) that are foreign to the host body (cells of grafted organs). K-cells and EK-cells (natural killer cells) belong to cell-killers. If the person has graft, the paracortical zone of the lymphatic node is widening.
When lymphocytes recognize antigens, cytotoxic T-cells interact with foreign cells, having molecules, including class I MHC (they are foreign graft cells) and the graft cells are destroyed. K-cells interact with antibodies applying to foreign cells and destroy the latter. EK-cells interact with cells having MHC and destroy these cells.
K-cells, EK-cells, and macrophages are not divided (multiplied) and do not undergo further differentiation when they meet antigens.
Plasmocytes and B-lymphocytes synthesize and release following antibodies (or immunoglobulins): IgG, IgM, IgA, IgE, and IgD. Most of the blood plasma immunoglobulin is IgG. The first immunoglobulin, which is released by the plasmocytes in immune response, is IgM. Immunoglobulin A (IgA) lodges in the mucous membranes. Its carbohydrate component is produced by the epithelial cells of the mucous membrane while its protein is produced by plasmocytes or lymphocytes. All immunoglobulins (except the IgD) are receptors of B-lymphocytes. IgA takes part in allergic reactions.
The Lymphatic Node Medulla
The medulla is pale. Interlacing medullar cords form it. The stroma of the medulla is made up of reticular tissue. The medullar cords include plasmocytes, B-lymphocytes, macrophages, and reticular cells. In the medullar cords there are capillaries (sinusoids).
Lymphatic Node Sinuses
Between the capsule and lymph nodules (follicles) there is the subcapsular sinus. Between the trabecula and lymph nodules there are cortical (perinodular) sinuses arising from the subcapsular sinus. Between the trabeculae and medullar cords there are medullar sinuses arising from the cortical sinuses. The medullar sinuses join to form the efferent lymphatic vessel leaving the lymphatic node through its hilus. Lining of the sinuses is endothelium (reticular endothelium). The endothelial cells are similar to the reticular cells. Amongst the endothelial cells there are macrophages engulfing antigens from the following lymph. The endothelial cells, lying on the sinus wall, applied to the trabeculae or capsule, rest on the basal membrane, while on the wall surface, applied to the lymph nodule or medullar cord, rest on the reticulum, formed by the reticular fibers. This facilitates passing of lymphocytes out or into sinuses.
Circulation of the Lymph through the Lymphatic
Nodes
The afferent lymphatic vessels, reaching the convex external surface of the lymphatic node, enter the subcapsular sinus. From this sinus a number of radial cortical sinuses run through the cortex towards the medullar sinuses. The latters drain into the efferent lymphatic vessel leaving the lymphatic node through its hilus. The efferent lymphatic vessels enter the larger lymphatic vessels, which drain into the thoracic or right lymphatic ducts, draining into cervical veins.
The Lymphatic Node Functions
The lymphatic nodes provide hemopoietic function i.e., entering lymphocytes undergo multiplication and antigen depending differentiation as a result effector lymphocytes and memory cells are formed. These cells take part in the immune reactions. It is called the immune defence function. The lymphatic node macrophages engulf bacteria and other harmful substances. It is also defence function. In addition to lymphocytes enter the lymph flowing through the lymphatic node as a result the lymph becomes rich with lymphocytes. In the lymphatic nodes the lymph is accumulated. In the lymphatic nodes of the mesentery lipids are stored.
The Lymphatic Node Blood Supply
The artery enters the lymphatic node through its hilus. It passes through the medulla to reach the cortex. Here the artery is bifurcated to form central and peripheral blood vessel plexuses. The central plexus supplies the lymph nodules (follicles) and medullar cords while the peripheral plexus supplies the trabeculae and capsule.
Age Dependent Changes of the Lymphatic
Nodes
The lymphatic nodes are fully developed in children of 3 years old. In elderly the connective tissue of the trabeculae and capsule grows till the size of the follicles and medullar cords decreases. The amount of lymphocytes decreases and the germinal centers of the lymph follicles disappear.
Hemal Lymphatic Nodes
In man these nodes are rare seen. They are located along the renal artery and aorta. Sometimes they are seen in the mediastinum. The hemal lymphatic nodes distinct from the regular nodes, they are smaller and the amount of lymph nodules is less than that of the regular lymphatic nodes. The hemal lymphatic nodes produce erythrocytes, granulocytes, monocytes, and platelets by the birth (sometimes after the birth) therefore their lymphatic node sinuses do not contain only lymphocytes, but erythrocytes, granulocytes, monocytes, and platelets as well.
The Spleen
It is the largest lymphoid organ in the body. Except of the hilus the surface of the spleen is covered by the peritoneum.
The Spleen Development
The spleen is derived from the cluster of the mesenchymal cells in the region of the mesentery root on the fifth week of the embryonic life. From the peripheral mesenchymal cells the capsule is formed. From the capsule trabeculae arise inwards the spleen bud. From the central mesenchymal cells the reticular stroma of the spleen is formed. On the twelfth week of the embryonic life the macrophages and stem cells enter the spleen bud. So hemopoiesis begins. Hemopoiesis is the most active on the fifth month of the embryonic life. Then it becomes less active and lasts until the birth. On the third month of the uterine life the sinusoids grow. The growing sinusoids divide the reticular stroma into the small islands. At first the islet hemopoietic cells are distributed equally around the artery then T-lymphocytes enter the region near this artery to form the thymus dependent zone. On the fifth month B-lymphocytes enter the space placed laterally the thymus dependent zone to form zone of B-lymphocytes. At the same time the amount of B-lymphocytes is in 3 times more than that of T-lymphocytes. On the sixth month of the embryonic life the red pulp is seen.
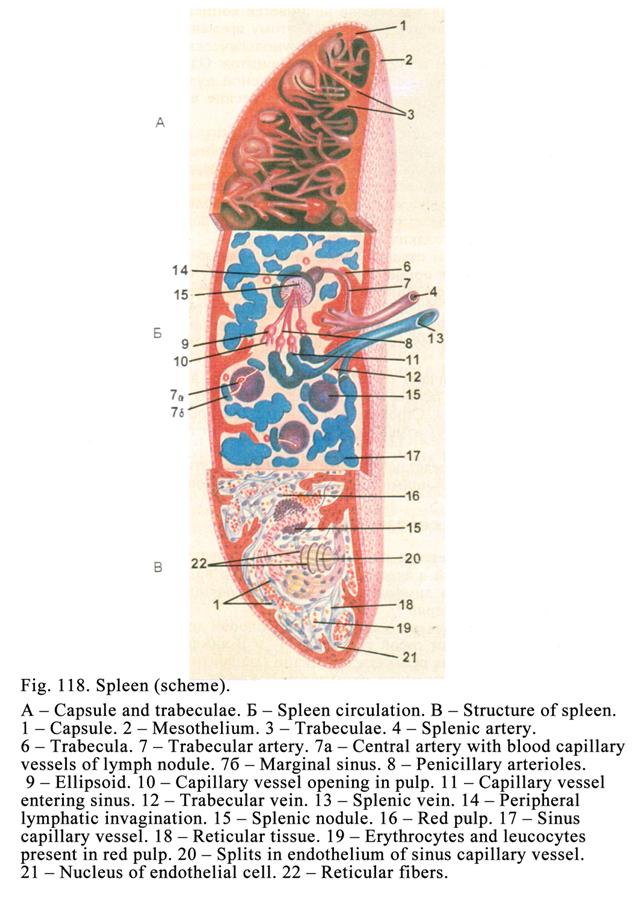
The Spleen Structure
The connective tissue capsule covers the spleen (Fig. 118), from which trabeculae is arised into the spleen substance. The capsule and trabeculae consists of the collagen and elastic fibers, connective tissue cells, and smooth muscle cells. The most of the muscle cells are in the capsule near the hilus. The capsule and trabeculae form the framework of the spleen. The spleen stroma is made up of reticular tissue containing reticular cells and reticular fibers. In the spleen there are the white and red pulps. About one fifth is the white pulp, about four fifths is red pulp.
The White Pulp
Splenic nodules and periarterial lymphatic vaginations represent the white pulp.
Splenic Nodules
The splenic nodules are sphered shaped (Fig. 119). They contain T-lymphocytes and B-lymphocytes, T-lymphoblasts and B-lymphoblasts, macrophages, dendritic cells, and dendritic antigen presenting cells. Trough the splenic nodule the lymph node artery passes. From this artery blood capillary vessels arise. They enter the marginal sinus of the splenic nodule. In the splenic nodule there are 4 zones: 1) periarterial zone (thymus dependent zone), 2) germinal zone (B-lymphocyte dependent zone), 3) mantle zone (mixed zone), and 4) marginal zone (mixed zone i.e., T-lymphocytes and B-lymphocytes zone).
The periarterial zone is similar to the paracortical zone of the lymphatic node i.e., it contains T-lymphocytes, T-lymphoblasts, and dendritic antigen presenting cells. In this zone T-lymphocytes, leaving the thymus, enter the spleen and undergo the antigen dependent differentiation as a result effector cells (helper T-cells, suppressor T-cells, cytotoxic T-cells, and memory cells) are formed. Then the effector cells pass into the blood capillary vessels and enter the marginal sinus of the lymph nodule, then enter the blood stream; at last they enter the connective tissue. Here these cells take part in the immune reactions.
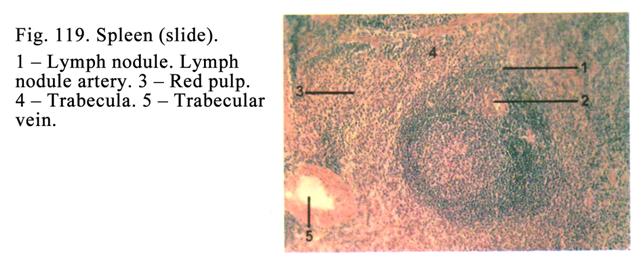
The germinal center (zone) is similar to the lymph nodules of the lymphatic nodes i.e., it contains B-lymphocytes, B-lymphoblasts, free macrophages, and dendritic cells. B-lymphocytes, produced in the bone marrow, enter the splenic nodules from the blood. In the germinal center B-lymphocytes undergo antigen dependent differentiation as a result they are converted into the effector cells (plasmocytes and memory cells). These cells are carried by blood to the connective tissue where they take part in the immune reactions.
The mantle zone consists of T-lymphocytes, B-lymphocytes, macrophages, memory cells, and reticular cells. This zone surrounds the periarterial and germinal zones.
The marginal zone is located around the mantle zone. It includes T-lymphocytes and B-lymphocytes (mixed zone). Its width is about 100 mm. Surround the marginal zone there is the red pulp.
Peripheral Lymphatic Vaginations
They are elongated. The lymphatic vaginations surround the white pulp arteries. They consist of two layers of lymphocytes: the external layer containing T-lymphocytes and the internal layer containing B-lymphocytes.
The Red Pulp
The reticular tissue, containing a number of blood vessels, predominantly sinus capillary vessels, forms the stroma of the red pulp. The spaces within the reticular tissue are filled particularly with erythrocytes. Between the sinus capillary vessels there are splenic cords containing plasmocytes, plasmoblasts, blood cells, and reticular cells.
The Spleen Blood Supply
The splenic artery (Fig. 118 4), entering the spleen, is divided into trabecular arteries entering the trabeculae. The trabecular arteries are the muscular artery types. The artery middle tunic consists of the smooth muscle cells staining deeper than the connective tissue of its external tunic and trabecula. Therefore the trabecular artery wall is well seen. The trabecular arteries branch into the red pulp arteries. The branches of the red pulp arteries reach the splenic nodules and enter them. The arteries passing through the splenic nodules are called the lymph node arteries, from which numerous capillaries arise in different directions. When the lymph node artery leaves the splenic nodules it branches into the penicillary arterioles. In the distal end each penicillary arteriole thickening, called the ellipsoid, is seen. The ellipsoid includes the reticular fibers and reticular cells. The ellipsoid serves as arterial sphincter regulating the blood influx into the spleen. If all the arteriolar sphincters are closed, the blood interflow is stopped. The penicillary arteriole part, which passes through the ellipsoid, is called the elliptical arteriole, from which numerous blood capillary vessels arise. Some capillary vessels open into the red pulp (it belongs to the open circulatory system) while other open into the sinus capillary vessels (it belongs to the closed circulatory system of the spleen).
Sinus Capillary Vessels
The vessels are 12-40 mm in diameter. Fenestrated endothelial cells line the sinus capillary wall. Trough the wall blood cells easily pass into the red pulp.
The sinus capillaries enter the red pulp veins. In places, where the sinus capillaries enter the red pulp veins, there are sphincters. When the sphincters are closed, the blood outflow from the spleen is stopped.
Trabecular Veins
The red pulp veins enter the trabecular veins. The trabecular veins are fibrous vein types (in the walls of this type there are no smooth muscle cells) therefore the wall of these veins does not distinct from the connective tissue of the trabecula. It helps to distinct the trabecular vein from the trabecular artery. The trabecular veins enter the splenic vein, which is the influx of the portal vein.
The Spleen Nerve Supply
In the spleen there are the effector nerve endings and receptors. The receptors are the terminals of dendrites of neurons present in the spinal nerve ganglions while effectors are the terminals of neurons located in the autonomic (sympathetic and parasympathetic) ganglions.
The Sympathetic Reflex Arc
The sympathetic reflex arc is a chain consisting of 3 neurons: 1) sensitive neuron of the spinal nerve ganglion, 2) associative neuron located in the spinal cord, and 3) efferent neuron in the sympathetic nerve ganglion.
Pathway of the Nerve Impulse through the
Sympathetic Reflex Arc
The stimulation is received by the receptor where the nerve impulse is produced. The nerve impulse travels along the cell dendrite to the cell body then to its axon. The axon (running with the posterior root of the spinal cord) contacts with the second neuron. The second neuron axon leaves the spinal cord (along with anterior root) and as preganglionic myelinated cholinergic nerve fiber is directed to the sympathetic nerve ganglion, where it contacts with the third neuron. The third neuron axon as the postganglionic non-myelinated adrenergic nerve fiber runs to the smooth muscle or other cells of the spleen.
The Parasympathetic Reflex Arc
The arc includes 3 neurons: 1) sensitive neuron of the spinal cord ganglion or the vagal nerve ganglion, 2) associative neuron in the spinal cord (in the lumbosacral segment) or in the vagal nucleus, and 3) neuron of the autonomic ganglion.
Pathway of the Nerve Impulse through the
Parasympathetic Reflex Arc
The stimulation is received by the receptor, which produces and transmits the nerve impulse to the first neuron (present in the spinal cord ganglion or the vagal ganglion) dendrite. The dendrite carries the impulse to the cell body then to the first neuron axon. The axon transmits the impulse to the second neuron located in the vagal nucleus or in the spinal cord. The second neuron axon, called the preganglionic myelinated cholinergic fiber, transmits the impulse to the third neuron located in the parasympathetic ganglion. The third neuron axon, called the postganglionic non-myelinated cholinergic nerve fiber, transmits the impulse to the smooth muscle cell or other cells.
The Spleen Functions
The spleen provides: 1) hemopoietic function (multiplication and antigen dependent differentiation of T-lymphocytes and B-lymphocytes), 2) protective function (phagocytosis and immune defence), 3) blood accumulation, and 4) break down of the old erythrocytes and platelets. At the same time the erythrocytes lose their osmotic stability and undergo haemolysis to form free hemoglobin. The hemoglobin splits to form substances containing the iron. These substances enter the circulation and travel to the bone marrow. Here the substances are engulfed by the macrophages. The macrophages give iron molecules to the developing erythrocytes.
Age Dependent Changes of the Spleen
In elderly the connective tissue of trabeculae and capsule grows and amount of the splenic nodules and their size decreases. The functional activity of the spleen becomes decreased.
The Spleen Regeneration
If 80% of the spleen mass is removed, it can be partially restored. The spleen stroma is restored due to division of the existing reticular cells. The hemopoietic cells are restored due to entrance of B-lymphocytes from the bone marrow and T-lymphocytes from the thymus.
Palatine Tonsils
Between the oral cavity and pharynx apart the palatine tonsils there are 2 tube tonsils, one lingual tonsil, one pharyngeal tonsil, and two laryngeal tonsils.
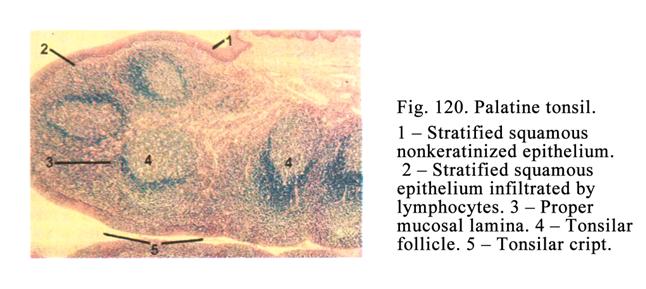
The Palatine Tonsil Development
The palatine tonsil begins to develop the 9-10th week of the embryo. In region of the palatine arcs the mesenchyme cell clusters are deposited to form the palatine tonsil buds (left and right). The oral cavity epithelium invaginates into the buds to form tonsil sinuses. Mesenchymal cells of the palatine tonsil buds undergo differentiation to form the reticular stroma of the tonsil. On the 17-18th week of the embryonic life in the tonsil buds the lymph follicles are seen. At the 19th week the amount of T-lymphocytes is about 60% while that of the B-lymphocytes is about 3%.
The Palatine Tonsil Structure
The palatine tonsils (Fig. 120) are located between the palatine arcs. The tonsils have oval form. The stratified squamous epithelium covers the tonsils. The epithelium extends into the tonsil substance to form 10-20 crypts.
In some places the epithelium is embedded by lymphocytes. It is called the lymphocytes infiltrated epithelium. The rest of epithelium, covering the tonsil, is called the epithelium non-infiltrated by lymphocytes. Through the infiltrated epithelium the lymphocytes pass into the crypt lumen or to the tonsil surface.
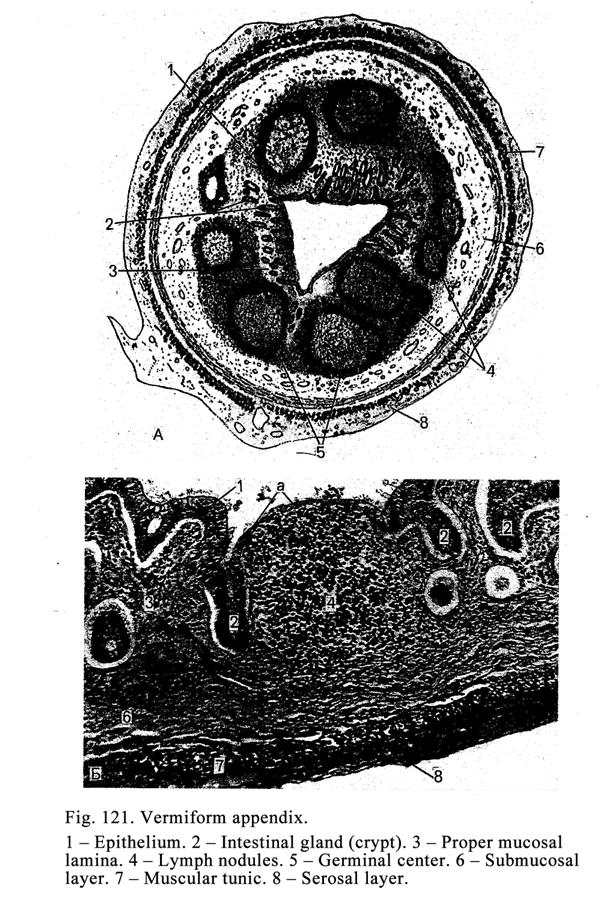
Subjacent to the epithelium there is loose connective tissue forming the proper mucosal lamina. In the lamina a number of lymph follicles are seen. The follicles contain T-lymphocytes, B-lymphocytes, macrophages and granulocytes. Multiplying lymphocytes are embedded into the surrounding connective tissue and epithelium, which can be destroyed by lymphocytes. The epithelium can be restored later.
Subjacent to the proper mucosal lamina the submucosal layer is located. This layer serves as the tonsil capsule separating the tonsil from deeper lying muscle tissue.
The tonsil functions are 1) hemopoiesis and 2) protective function (phagocytosis and immune defence).
Mucosa Associated Lymphoid Tissue in the
Alimentary System
Small collections
of lymphoid tissue similar to the lymph follicles of the lymphatic nodes may be
seen anywhere along the length of the gut. The lymphoid tissue or follicles increase
in number and size along the small intestine being the most numerous and
largest in the large intestine. The follicles consist of T-lymphocytes,
B-lymphocytes, and macrophages. They are
The vermiform appendix (Fig. 121) contains a number of follicles so it is called the intestinal tonsil. The lymph follicles provide 1) hemopoietic function and 2) defence function.
Lymphocytopoiesis of T-lymphocytes
In the bone marrow the precursors of T-lymphocytes develop (CFU-LT), which enter the thymus.
Lymphocytopoiesis in the Thymus
The first cell is precursor of T-lymphocyte, the second cell is T-lymphoblast, the third cell is middle T-lymphocyte, and the fourth cell is T-lymphocyte.
Lymphocytopoiesis of T-lymphocytes in the
Spleen, Lymphatic Nodes, and Lymph Nodules
The first cell is T-lymphocyte, the second cell is T-lymphoblast, the third cell is middle T-lymphocyte, and the fourth cell is T-lymphocyte (effector and memory cells).
Lymphocytopoiesis of B-lymphocytes in the
Bone Marrow
The first cell is precursor of B-lymphocyte (CFU-B), the second cell is B-lymphoblast, the third cell is middle B-lymphocyte (prelymphocyte), and the fourth cell is B-lymphocyte.
Lymphocytopoiesis of B-lymphocytes in the
Spleen, Lymphatic Nodes, and Lymph Follicles
The first cell is B-lymphocyte, the second cell is plasmoblast, the third cell is middle plasmocyte (preplasmocyte), and the fourth cell is plasmocyte (effector and memory cells).
CHAPTER
19
ANTERIOR
PART OF THE ALIMENTARY APPARATUS
The digestive apparatus (Fig. 122) includes 3 parts: anterior, middle, and posterior. The anterior part consists of the oral cavity and all organs relating to it and the oesophagus. The anterior part is concerned with mechanical and partly chemical treating of the food (mixing and blending).
The middle part includes the stomach, small and large intestines, liver, and pancreas. The middle part predominantly produces chemical treating and absorption of the food.
The posterior part includes the anal canal removing feces.
Development of the Wall Gut Tissues
The epithelium of the mucosal layer of the oral cavity, esophagus, and the lower part of the anal canal is derived from the ectoderm. The epithelium of the middle part of the gut is derived from the endoderm. The connective and smooth muscle tissues of the gut wall are derived from the mesenchyme. The mesothelium of the serosal layer is derived from the mesoderm.
The Wall Gut Structure
The wall gut made of 4 layers: 1) mucosal layer, 2) submucosal layer, 3) muscular layer, and 4) serosal layer (in some places adventitial layer).
The mucosal layer of the oral cavity consists of 2 layers while the rest gut part consists of 3 layers: 1) epithelium, 2) proper mucosal lamina, and 3) muscular mucosal lamina.
The Epithelium
The epithelial lining in the anterior and posterior parts of the gut is stratified squamous, in the middle part is simple columnar. The glands of the gut can be
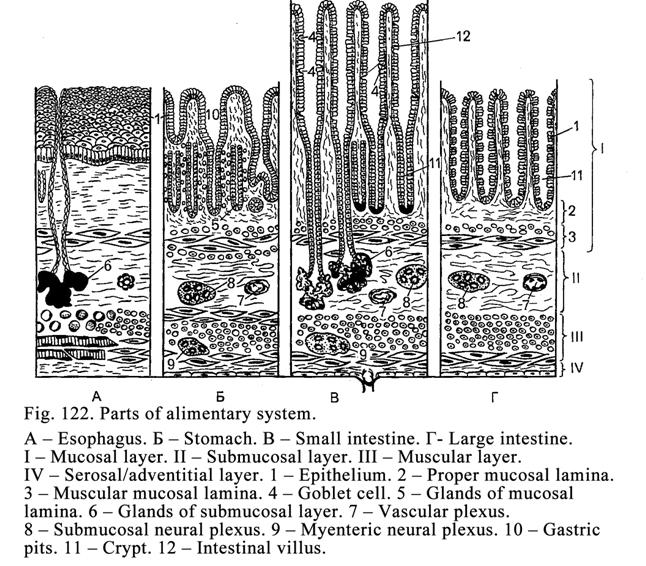
localized in the epithelial layer (goblet cells in the small and large intestines). They can be located in the proper mucosal lamina (esophageal cardiac glands, glands of the stomach, and crypts of the small and large intestines). The glands can be located in the submucosal layer (proper esophageal glands and duodenal glands). There are glands outside the gut (liver and pancreas).
Among the epithelial cells and in the glands there are single endocrine cells releasing serotonin, melatonin, gastric encephalin, and others hormonal substances. The most number of the endocrine cells are in the middle part of the gut.
The Proper Mucosal Lamina
The lamina lies deep to the basal membrane of the epithelium. It consists of loose connective tissue. In the lamina simple glands (esophagus, stomach), blood and lymphatic vessels, nerves, and lymph nodules are seen.
The Muscular Mucosal Lamina
It consists of 1-3 layers. In the esophagus there is only 1 layer, in the small and large intestines there are 2 layers, in the stomach there are 3 layers.
The Mucosal Layer Surface
The mucosal layer may be various in different pats of the alimentary system. In the oral cavity it is smooth, in the stomach there are fovea (gastric pits), in the small intestine villi and crypts are seen, in the large intestine only crypts are seen. The folds of the proper muscular lamina are in all the gut parts.
The Submucosal Layer
The submucosal layer is made up of the loose connective tissue, in which plexuses (arterial, venous, nervous, and lymphatic) and lymph nodules (follicles) are found. In some places of the gut (esophagus and duodenum) the submucosal layer includes glands.
The Muscular Tunic
The muscular tunic
includes 2 layers (in the stomach wall there are 3 layers). The internal layer is
the circular layer while the external one is the longitudinal layer. Between
the layers there is the loose connective tissue.
The Serosal Layer
The serosal layer covers the esophagus lower part and the middle part of gut. It consists of the proper connective tissue layer covered by the mesothelium.
The Adventitial Layer
The adventitial layer covers the esophagus upper part and anal canal lower part. This layer consists of the loose connective tissue.
The Gut Wall Blood Supply
The blood supply of the gut wall is provided by the arterial and venous plexuses located in the mucosal and submucosal layers of the gut wall. In addition such plexus is found in the small intestine wall muscular tunic layer. The most powerful arterial and venous plexuses are in the submucosal layer. Between the plexuses there are anastomoses. In the gut wall there are prominent arteriovenous anastomoses. Deep to the basal membrane of the epithelium are sub epithelial dense capillary network surrounding glands, crypts, and enter the small intestine villi.
Gut Wall Lymphatic Vessels
All layers of the gut wall have lymph capillary vessels. The largest lymphatic vessel plexus is in the submucosal layer of the gut wall. In the small intestine the lymph capillary vessels enter the intestinal villi.
The Gut Wall Nerve Supply
The efferent and afferent nerve fibers, forming the nerve plexuses, provide innervation of the gut wall. The efferent nerve fibers are sympathetic and parasympathetic ones. The sympathetic nerve fibers are axons of the sympathetic ganglion neurons. The parasympathetic nerve fibers are the axons of the parasympathetic ganglion (located within gut wall) efferent neurons (Dogels I type cells). The efferent nerve endings terminate in the smooth muscle cells or gut wall glands.
The afferent nerve fibers are dendrites of the sensitive neurons located in the spinal ganglion or in the ganglion of intrinsic neural plexus. The dendrites (sensitive nerve fibers) end by complicate receptors, which may simultaneously innervate smooth muscle cells of blood vessels, gut wall muscular tunic, muscular mucosal lamina, and epithelium.
The Alimentary System Anterior Part
The anterior part of the alimentary system includes the oral cavity and the esophagus. The oral cavity includes lips, cheeks, gums, tonsils, salivary glands, and teeth.
The Oral Cavity
The oral cavity provides chewing and partly chemical processing of the food. The oral mucosal tunic consists of 2 layers: 1) stratified squamous epithelium and 2) proper mucosal lamina. The epithelium provides protective (barrier) function. Deep to the basement membrane there is proper mucosal lamina consisting of loose connective tissue. Here there is an abundant capillary vessel network, lymph nodules constituting tonsils providing hemopoietic and defence functions.
The Lips
The lips (Fig. 123) include 3 parts: 1) skin part, 2) intermediate part, and 3) mucosal part.
The skin part is covered by epidermis. Deep to the epidermis there is connective tissue; its short papillae extend into the epidermis. Here hair roots, sebaceous and sweat glands are seen.
The Intermediate Part
The part includes the external and internal zones.
The external zone is covered by the thin stratified squamous epithelium. The subjacent layer consists of loose connective tissue giving short papillae into the epithelium. In the connective tissue there are a few sebaceous glands while the hair roots and sweat glands are absent
The internal zone is covered by thick stratified squamous non-keratinized epithelium. The underlining loose connective tissue gives off high papillae into epithelium. Here the sweat and sebaceous glands are absent. On the internal zone of child epithelium villi are seen. These villi help to hold the nipple of breast at time of the feeding. In the internal zone there are numerous nerve endings.
The Mucosal Part
The mucosal part of the lip is covered by the mucosal layer consisting of 2 laminae: 1) the epithelial lamina (stratified squamous non-keratinized epithelium), and 2) proper mucosal lamina, from which short papillae invade the epithelial lamina. The loose connective tissue, included into the proper mucosal lamina, is continuous with the loose connective tissue of the submucosal layer. In the latter there are terminal regions of the composed tubular-alveolar glands of the lips. Ducts of these glands open on the mucosal layer surface. Deep to the submucosal layer the oral muscular layer lies.
The Cheek
The cheek has 3 parts: 1) maxillary part, 2) mandibular part, and 3) intermediate part.
The maxillary part of the cheek and the mandibular part of that have the identical structure. Their mucosal layers contain 2 laminae: 1) epithelial (stratified squamous non-keratinized epithelium) and 2) proper mucosal lamina, consisting of loose connective tissue, from which short papillae invade the stratified squamous epithelium.
The deeper layer is the submucosal layer, consisting of loose connective tissue, in which there are a number of cheek glands. The largest of them is the gland located opposite the molar. Far from the mouth angle the glands, invade the connective tissue, located between the muscle tissues.
The intermediate
part of the cheek is similar to the internal zone
of the lip. Its width is about
The Gingiva (Gum)
It applies to the jawbone and partly to the tooth surface. The mucosal layer of the gum consists of the stratified squamous epithelium (sometimes keratinized) and proper mucosal lamina. The latter consists of 2 layers 1) papillary layer and 2) reticular layer, its collagen fibers attach to the jaw bone and the tooth neck surface to form the fixed region of the gingiva. The gingiva epithelium also attaches to the tooth neck. The gingiva edge, which does not attach to the tooth, is called the free gingiva region.
Between the free
region and the tooth surface there is a space called the gingival sulcus. Its
depth is 1-
The Hard Palate
This consists of the bone covered by the mucosal layer. The layer is made up of the epithelial lamina (stratified squamous epithelium) and proper mucosal lamina, consisting of the connective tissue, from which short papillae extend into the epithelium. The epithelium may be partly keratinized. In the region of the palate suture and the place, where the hard palate is continuous with the gingiva, its proper mucosal lamina fuses with the bone periosteum i.e., in these places there are no submucosal layer. In the anterior part of the hard palate between the proper mucosal layer and periosteum there is a fat. This part is called the adipose zone. In the posterior part of the hard palate there are salivary glands. This part is called the glandular zone.
The Soft Palate
The soft palate and palatine uvula consist of the fascicle (cord), containing the tendons and striated muscles, surrounded by submucosal and mucosal layers. The soft palate and palatine uvula have 2 surfaces: 1) oral-pharyngeal and 2) nasal-pharyngeal surfaces. The mucosal layer of the oral surface is covered with stratified squamous non-keratinized epithelium while the nasal surface is covered with the pseudostratified epithelium. The boundary of uvula between both epithelia of the newborn is on the lateral surface while in adult the stratified squamous epithelium surrounds entire palatine uvula.
The proper mucosal layer of the oral-pharyngeal surface consists of the loose connective tissue, from which high papillae extend into the epithelium. Deep to the mucosal layer the submucosal layer lies in which the terminal regions of the salivary glands are seen.
Between the myofibers of the striated muscle tissue of the palatine uvula there are anastomoses.
The nasal-pharyngeal surface of the soft palate is covered with the mucosal layer consisting of 2 laminae: 1) pseudostratified epithelium and 2) proper mucosal lamina represented by loose connective tissue devoid of the papillae. In the proper mucosal lamina mucous salivary glands are present. In the pseudostratified epithelium of the mucosal lamina there are ciliated cells, goblet cells, and non-differentiated cells.
The Tongue
The tongue (Fig. 124) may be divided into its body, root and tip. It is covered with the mucosal layer, which is strongly fused with the aponeurosis of the tongue striated muscle on its dorsal and side surfaces. The mucosal layer consists of 2 laminae: 1) stratified squamous epithelium (partly keratinized) and 2) proper mucosal lamina. On the tongue dorsal part there are 4 special papilla types: 1) filiform papillae, 2) fungiform papillae, 3) foliate papillae, and 4) vallate papillae.
Every papilla has the core (extending of the connective tissue of the proper mucosal lamina). In the core the primary connective tissue papillae and some secondary (2-20) papillae arising from the primary papillae may be seen. The connective tissue core is covered by the stratified squamous epithelium, which may be keratinized in some papilla types. In connective tissue core of the papilla there are capillary vessels.
Filiform
papillae are the most numerous and smallest ones.
They are scattered on the dorsal surface of the tongue. Their length is about
Fungiform
papillae are scattered amongst the filiform
papillae. They have a narrow lower part and a wide apical part. The papillae
are 0.7-
Foliate papillae
are only in children. They arrange along the
lateral margins of the tongue on its dorsal surface to make 4-8 rows. They are
2-
Vallate papillae
arrange between the body and the root of the tongue
to have V-like appearance. Their amount is 6-12. They are 1-
Thus, the filiform papillae distinguish from the rest papillae by 2 signs: 1) they are covered with stratified squamous keratinized epithelium (the rest papillae are covered with stratified squamous non-keratinized epithelium) and 2) taste buds in them are absent.
The Mucosal Layer of the Ventral Aspect of
the Tongue
The mucosal layer consists of 2 laminae: 1) stratified squamous non-keratinized epithelium and 2) proper mucosal lamina consisting of loose connective tissue. Deep to the proper lamina the submucosal layer lies. Due to the submucosal layer the mucosal layer is mobile. The tongue lies on the oral cavity floor. Between the tongues tip of the ventral surface and oral cavity floor there is a thin ligament.
The Tongue Root Mucosal Layer
In the mucosal
layer of the tongue root the papillae are absent. Here there are sockets and
elevations. In the elevations there are some lymph nodules. They are
The Muscle Tissue of the Tongue
The tongue substance is made up chiefly of skeletal muscles. The muscle fibers are arranged in bundles, which run in vertical, transverse, and longitudinal directions. Between the muscle bundles there are some connective tissue and terminal regions of lingual glands. The substance of the tongue is divided into right and left symmetrical halves by the connective tissue septum. The aponeurosis of the lingual musculature is made up of interlacing collagen fibers forming the reticular layer. The proper mucosal lamina of the tongue dorsal part is attached to the reticular layer. The tongue muscle tendons, passing through the aponeurosis loops, fasten to the collagen fiber bundles of the proper mucosal layer.
Lingual Glands
The lingual glands are divided into serous, mucous and mixed (hybrid) glands.
The serous glands locate near the vallate and papillae foliate in the depth of the tongue. Their ducts open into the groove of the vallate papilla and into the spaces between the papillae foliate. Those are simple tubular branching glands.
The mucous glands are simple tubular-alveolar branching glands. They arrange along the tongue marginal surface and the tongue root. Their ducts open into lingual tonsil crypts.
The mixed glands are present on the ventral aspect of the tongue apex (tip). They contain both mucous and serous acini. Their ducts open on the ventral tongue aspect.
The Tongue Blood Supply
The blood supply of the tongue provides by lingual arteries branching within connective tissue and locating between muscle bundles. The tongue arteries give off branches towards the dorsal surface of the tongue to form horizontal lingual arterial plexus. From the plexus arterioles arise. The latter ramify to form capillary vessel plexuses in tongue papillae. From the tongue dorsal part the blue blood outflows into the venous plexus located in the mucosal layer of the tongue. In the ventral aspect of the tongue there is a large venous plexus.
Small lymphatic vessels form the mucosal lymphatic plexus and a plexus near the tonsil. From here the lymph outflows into the submucosal lymphatic plexus (in tongue ventral aspect).
The Tongue Nerve Supply
Branches of the hypoglossal nerve and tympanic nerve provide the efferent innervation of the tongue. The afferent innervation of the two third of the tongue anterior part is provided by the branches of the trigeminal nerve. Branches of the glossopharyngeal nerve provide the afferent innervation of one third of the posterior part of the tongue. Nerve fibers form the inter-muscular nerve plexus, from which efferent nerve fibers go to the blood vessels and muscles. The afferent nerve fibers reach the taste buds, epithelium, and others tongue structures.
The tongue provides 1) mechanical function (food mixes), 2) it takes part in swallowing, 3) it is a gustatory organ, and 4) is necessary for speech.
Large Salivary Glands
Apart the small glands (lip, cheek, and lingual glands) large gland ducts open into the oral cavity. There are 3 pairs of large salivary glands: 1) parotid, 2) submandibular, and 3) sublingual.
Development of the Large Salivary
Glands
The buds of parotid (right and left) glands are formed at 8th week of embryonic life. At the same time epithelial cords extend into the subjacent mesenchyme and direct towards the external acoustic meatus to form the terminal regions and ducts of the parotid. The capsule and connective tissue stroma of the gland is formed from mesenchyme. At 10-12th week of the uterine life nerve fibers extend into the gland buds. At 18-24th week in the gland buds terminal regions are formed. At the 32-36th week of the uterine life in the terminal region the lumen is seen.
The submandibular salivary gland buds (right and left) are formed at 6th week of the embryonic life from the oral cavity epithelium. The epithelial cords, extending into subjacent mesenchyme, convert into the epithelial ducts and terminal regions at 16th week of the uterine life. The terminal region of the glands may be serous and mixed (contain serous and mucous cells). The terminal region mucous cells are formed from intercalated duct cells. The development of the submandibular glands lasts by the embryonic life end and after the embryonic life.
The buds of the sublingual glands are formed from the epithelial cords separating from the buds of the submandibular glands at 8th week of the embryonic life.
The Large Salivary Gland Structure
Every gland is covered with connective tissue capsule, from which trabeculae arise. The trabeculae divide the gland into lobules. The lobules contain terminal regions and intralobular ducts including intercalated and striated ducts (Fig. 125).
Every large gland contains its own terminal regions. The parotid contains only serous terminal regions. The submandibular gland contains serous and mixed terminal regions. The sublingual gland contains serous, mixed, and mucous ones.
In the trabeculae blood vessels, lymphatic vessels, nerves, and interlobular ducts are seen. The striated ducts enter the interlobular duct. The interlobular ducts run into the gland duct entering the oral cavity.
Parotid Glands
These are the largest salivary glands. They are covered with the connective tissue capsule, from which trabeculae arise to divide the glands into lobules. Every lobule includes the serous terminal regions, intercalated and striated ducts. The parotid is the compound alveolar gland releasing the serous secretion.
The serous terminal regions of the gland have round or oval shape. They consist of 1) serous glandular cells and 2) myoepithelial cells. Between the terminal regions there is some thin connective tissue forming the glandular stroma.
The serous cells have conical shape. Their wide basal ends lie on the basement membrane while apical parts direct towards the lumen. Between the cells there are intercellular canaliculi, in which the secretion releases. From here the secretion runs into the terminal region lumen. The cell nucleus, lying in the cell basal part, is round or oval. In the cells there is the prominent Golgi complex, rough ER, and mitochondria. In the apical part of the cell secretory granules, containing predominantly serous secretion, are seen. Their cell cytoplasm is slightly basophil.
The myoepithelial cells are branched (dendritic cells) locating between the basal membrane and serous cell basal parts. The cell contraction provides the secretion releasing from the serous cells into the terminal region lumen and duct.
The intercalated ducts are the smallest ducts. They arise from the terminal regions. The duct walls consist of the layer of cuboidal or flat epithelial cells and myoepithelial cells. In the parotid these ducts develop well. They branch. The intercalated ducts run into the striated duct.
The striated ducts of the parotid are conspicuous. They have a large diameter and a wide lumen. The lumen is lined with the simple columnar epithelium. Between the epithelium and basal membrane myoepithelial cells are seen. The apical part of the striated duct cells bears microvilli while on their basal part folds of the cell membrane are seen. Round nucleus of the cell is placed in its basal part. The cell oxyphil cytoplasm contains Golgi complex, mitochondria. In its apical part secretory granules are seen. The striated ducts run into the interlobular ducts.
The interlobular ducts locate in intralobular trabeculae. Two layers of cuboidal epithelium line their initial part while their end is lined with the stratified cuboidal epithelium. The interlobular ducts run into the glandular duct.
The gland duct initial part is lined with the stratified cuboidal epithelium while its end is lined with the stratified squamous non-keratinized epithelium. The duct perforates the cheek muscle and opens into the oral cavity vestibule opposite the second upper molar.
The Submandibular Salivary Glands
These are compounding tubular-alveolar glands locating under the mandibular and covered with the connective tissue capsule, from which the connective tissue trabeculae arise. The trabeculae divide the gland into lobules. Every lobule is made up of the serous and mixed terminal regions, intercalated and striated ducts. The serous terminal region structure is similar to that of the parotid.
The mixed terminal regions consist of mucous, serous, and myoepithelial cells. The serous cuboidal cells arrange surround the mucous cells to form the serous demilunes (semilunar serosa).
The semilunar serosa consists of cuboidal serous cells. Between them there are intercellular canaliculi. The cell basal end lies on the basement membrane their apical part is attached to mucous cells. The slightly basophil cell cytoplasm includes a round nucleus and such organelles as Golgi complex, mitochondria, and rough ER.
The mixed terminal region mucous cells locate in the terminal region center. They have conic shape and light cytoplasm. Between the cells there are intercellular canaliculi. The cell basal part directs towards the semilunar serosa the apical part of the cells direct towards the terminal region lumen. The mucous cell flattened nuclei locate in the cell basal part. The nuclei contain the dense chromatin (heterochromatin) the nucleoli are absent i.e., the nuclei are inactive. The nuclei are inactive because the cells do not synthesize protein. The nucleus is known to regulate the protein synthesis. Because these sells only synthesize the mucous secretion the regulating function of the protein synthesis is not necessary. The mucous cell cytoplasm contains the Golgi complex, mitochondria, and smooth ER. In the cell apical part mucous granules are seen.
The myoepithelial cells of the mixed terminal regions are located between the basement membrane and semilunar serosa cells. The myoepithelial cells take part in releasing of the secretion from the glandular cells and ducts.
The intercalated ducts of the submandibular glands are developed weakly they are short and not branching. It is because that at the time of embryonic life the part of the duct cells has converted into mucous cells of the mixed terminal regions.
The striated ducts of the submandibular glands are prominent and have dilations. In the duct wall the tall light cells, wide dark cells, goblet cells, and conic non-differentiated cells are present. In the striated ducts of some vertebrates there are fragments, which cells contain prominent Golgi complex, ER, and mitochondria.
These cells produce some hormones (growth factor, insulin-like factor and others). The striated ducts run into interlobular ducts.
The proximal part of the interlobular ducts consists of two layers of cuboidal epithelial cells while their distal part is lined with stratified cuboidal epithelium. The interlobular ducts run into the gland duct.
The stratified cuboidal epithelium lines the initial part of the gland duct while its distal part is lined with stratified squamous non-keratinized epithelium. It opens on the ventral surface of the tongue.
Sublingual Salivary Glands
These are the smallest salivary glands. They are covered with the capsule and divided into lobules by trabeculae arising from the capsule. The lobules contain 3 types of terminal regions: 1) serous, 2) mixed, and 3) mucous. The serous and mucous terminal regions are similar to those of the parotid and submandibular glands.
The mucous terminal regions consist of conic mucous cells and myoepithelial cells. Between slightly staining mucous cells there are intercellular canaliculi. In the flattened cell nuclei, containing rough chromatin clusters, nucleoli are absent. In the cell cytoplasm there are smooth ER, mitochondria, and Golgi complex. In the cell apical part the mucous secretory granules are seen. These cells synthesize and release the mucous secretion. Between the mucous cells and basement membrane there are myoepithelial cells.
The intercalated ducts of the sublingual glands are weakly developed because the part of the duct cells has converted into mucous cells of the mucous and mixed terminal regions at the time of embryonic life.
The striated ducts of the sublingual glands are weakly developed to comparison with those of the parotid and submandibular glands. The ducts run into intralobular ducts.
The internal part of the interlobular ducts consists of two layers of cuboidal epithelial cells while the distal part of the ducts is lined by stratified cuboidal epithelium. The ducts run into the gland duct.
The gland duct wall proximal part consists of the stratified cuboidal epithelium but its distal part is lined by stratified squamous epithelium. The gland duct opens on the ventral surface of the tongue near that of the submandibular gland.
Blood Supply of the Large Salivary Glands
Arteries, entering the salivary gland, branch into the lobular arteries; running along with the interlobular ducts, divide into the intralobular arteries, running together intralobular ducts. The intralobular arteries branch to form capillary network surrounding the terminal regions. The capillary vessels run into venules going into the intralobular veins. The intralobular veins run into the interlobular veins. Between the arterioles and venules on the level of the intralobular vessels and before the capillary network there are arteriovenous anastomoses. When the anastomoses close, the blood flows into the capillary vessels so the secretion releases from terminal regions rapidly.
The Nerve Supply of the Large Salivary
Glands
The innervation of the large salivary glands is provided by both sympathetic and parasympathetic nerve fibers ending by motor terminals on smooth muscle cells of blood vessels and neurosecretory ends on the glandular cells. The impulses, traveling in the sympathetic nerve fibers, provide releasing of the viscous saliva while the impulses traveling in the parasympathetic nerve fibers provide releasing of the liquor saliva, i.e. serous secretion.
Age Dependent Changes of the Large
Salivary Glands
The parotid releases the mucous secretion by the age of 2 and afterwards it releases the serous secretion. At the age of 80 it again releases the mucous secretion.
The serous and mixed terminal regions of the submandibular gland end their development at the age of 6 months. But it continues growing by the age of 25. After it at the age of 50 the gland undergoes an involution.
The most active development of the sublingual gland is noted at the age of 2 (the parotid and submandibular glands also rapidly grow at the age of 2).
The large salivary glands provide 1) exocrine function, 2) excretory function, and 3) endocrine function.
The exocrine functions is releasing of the saliva. The saliva consists of the water (99%), proteins, enzymes, salts, and cells (epithelial cells and leucocytes). The saliva moistens the food, takes part in the initial chemical breakdown of the food, and facilitates food digestion.
The saliva includes various enzymes. The carbohydrate splitting enzymes are amylase, maltase, and hyaluronidase. The nucleic acid splitting enzymes are nuclease and kallikrein. The protein splitting enzymes are kallikrein-like proteases, trypsin, and pepsin. In addition the saliva contains lysozyme, which destroys bacteria.
The excretory function is releasing of the urine acid, urea, creatin, iron, iodine, and others substances. As the endocrine glands the salivary glands discharge the insulin-like factor, the nerve growth factor, the epithelium growth factor, and others factors.
CHAPTER
20
TEETH
& OESOPHAGUS
The teeth present in the oral cavity and the esophagus belong to the anterior part of the alimentary system.
Teeth
In the oral cavity of an adult there are usually 32 teeth. In every jaw half (right and left) there are 8 teeth (2 cutting teeth, 1 canine tooth, 2 premolars, and 3 cheek teeth). The third cheek tooth is called the wisdom tooth.
In a child (at the age of 6) oral cavity there are 20 deciduous teeth. Every jaw half contains 5 teeth (2 cutting teeth, 1 canine tooth, and 2 molars or cheek teeth).
The tooth consists of the crown, which is seen in the mouth, the cervix, covered by the gum, and the root embedded in the socket in the bone of jaw. The tooth is made up of hard and soft tissues. The hard tissues are the enamel, dentine, and cement. The soft tissue is the pulp. In the socket the tooth root is fixed by supporting apparatus including cement, periodontium, and the bone wall of the socket.
Development of Teeth
There are 3 stages of the development of teeth: 1) formation of the tooth lamina and tooth germs, 2) differentiation of the tooth germs, and 3) formation of the tooth tissues (Fig. 126).
The First Stage (Fig. 126A)
At the 6-7th week of the embryonic life the vestibular lamina forms, which consists of the oral epithelium, embedding into the oral cavity marginal mesenchyme. After it the lamina splits to form the vestibule of the oral cavity. Lateral and medial walls confine the vestibule. Then the medial wall epithelium extends into the subjacent connective tissue to form the dental lamina (there are 2 laminae: upper and lower). 10 tooth buds grow from the internal surface of the lamina. Mesenchyme extends into every bud to form the dental papilla (Fig. 126. 3), which becomes more and more as a result the tooth bud converts into the enamel organ. The mesenchyme, surrounding the enamel organ, becomes dense to form the dental saccule. The enamel organ, dental papilla, and dental saccule constitute the tooth germ.
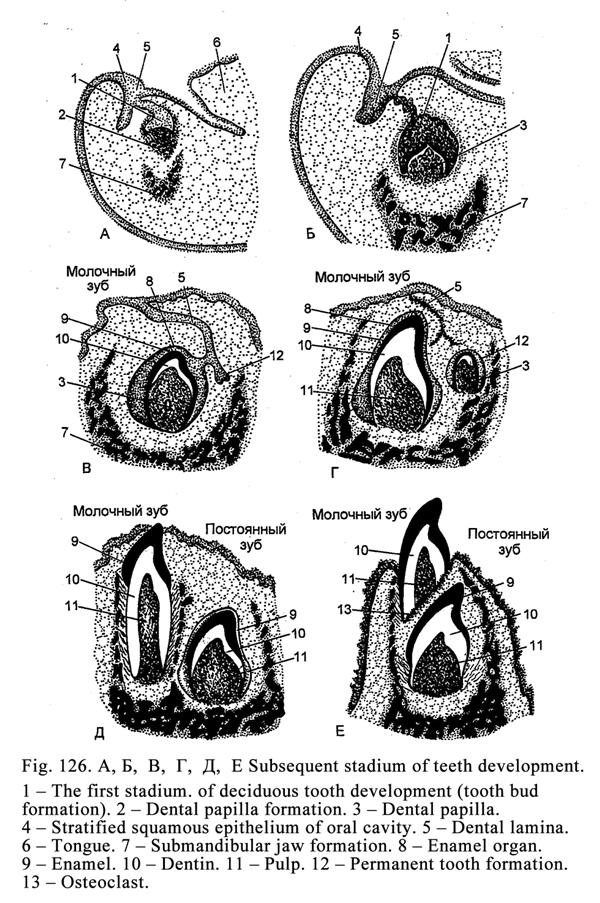
The Second Stage
At the time of the differentiation of the enamel organ (or dental organ) 3 layers are formed: internal, external, and mediate one.
The internal layer is attached to the dental papilla. Its epithelial cells, called ameloblasts, are column shaped. From these cells the enamel is derived.
The external layer cells of the dental organ (Fig. 126B 8) are flat they attached to the dental saccule. Some of these cells are reduced; some of them fuse with gum epithelium after the tooth eruption. Between the internal and external cells there is an intermediate layer called the stellate reticulum (its cells are star-shaped). Between these cells there is some fluid. After the tooth eruption the enamel cuticle will be formed from these cells.
The dental organ is connected to the dental lamina by the neck. At the third month the dental organ separates from the lamina.
The dental papilla (FIG. 126B 3) also undergoes differentiation. It grows and grows and extends into the dental organ deeper. The vessels and nerves enter the dental papilla. Its superficial mesenchymal cells differentiate into precursors of dentinoblasts. Later the dentine will be formed by these cells. The central mesenchymal cells of the dental papilla differentiate into the connective tissue cells, which later will create the dental pulp.
At time of the
stage differentiation the dental saccule is divided
into 2 layers: 1) internal and 2) external layers. From the internal layer
later cement will develop, from the external layer will periodontium develop.
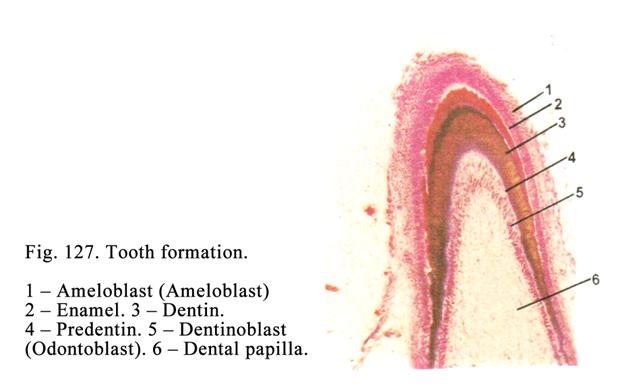
The third stage
The dentine (Fig. 126B 10 & Fig.127. 3) formation begins at the fourth month of the embryonic life. The dentinoblast precursors convert into dentinoblasts. The dentinoblasts are about 20 mm in length and 8 mm in diameter. They contain an oval or round nucleus, Golgi complex, Rough ER, mitochondria, and alkaline phosphatase. The dentinoblasts give off the central process directing towards the dental papilla.
The dentinoblasts produce the collagen molecules, from which the collagen fibers are formed. They also produce the glue substance (glycoproteins, proteoglycans and others substances). At first the collagen fibers have radial direction to form the mantle predentin. When the mantle predentin thickness becomes 40-80 mm, the following collagen fibers have longitudinal direction to form the juxstapulpar predentin.When the predentin becomes thicker and thicker dentinoblasts move towards central part of the dental papilla.
At the same time the dentinoblast peripheral process ends stay at the same place and become longer and longer (because it grows). The predentin is made around the processes to form dental tubules. The predentin is a soft substance. It may be cut by a knife. The predentin must be mineralized to convert into dentin (hard substance).
At the fifth month of the embryonic life the predentin undergoes mineralizing. At the same time dentinoblasts release the alkaline phosphatase splitting the complexes consisting of carbohydrates and phosphoric acid so the result is that the phosphoric acid becomes free. The phosphoric acid combines with the calcium to form the calcium phosphate. The calcium phosphate deposits in the predentin to form numerous small focuses. The focuses grow to form spherical structures. The structures fuse with each other resulting in the hard dentin formation.
The peripheral predentin parts do not mineralize to form irregular non-mineralized spaces called the interglobular spaces. In addition the predentin part, which is attached to the pulp, does not undergo the mineralizing. Therefore it is called the juxtapulpar predentin.
The dental pulp is formed simultaneously with the dentin formation. The dental papilla central cells convert into fibroblasts producing collagen molecules and other components of the pulp intercellular substance.
The enamel is formed (Fig. 127. 2) after the dentine formation. At the same time the passage of nutrients from the dental papilla to the ameloblast stops because between the dental papilla and ameloblasts the dentin has formed. It leads to the inversion i.e., the nucleus, Golgi complex, and cytocenter travel from the basal part of the ameloblast to its apical part and the basal part, directing to the dental papilla, becomes the apical part while the apical part, directing to the stellate reticulum, becomes the basal part of the cell. Thereafter the nutrients pass to ameloblasts from the stellate reticulum (intermediate layer). Thus, the dentine formation stimulates the inversion of the ameloblast after it the enamel is formed. On the rough ER of the ameloblasts the protein is synthesized. The protein granules move towards the apical part of the ameloblast. In this part the protein granules accumulate to form enamel cuticle plate. The cuticle plate becomes long because the granules move and move into it. Therefore the ameloblast becomes shorter and shorter and retreats to periphery. The long cuticle plate undergoes mineralizing to form the enamel prism. At the same time the dentin external surface undergoes the resorption so it assumes non-even aspect and to that the enamel close joins with the dentin.
The cement is formed at the age of 6 (month) when teeth erupt and dental roots are formed.
Structure of Teeth
The tooth includes enamel, dentin, cement, and pulp.
The Enamel Structure
The enamel covers
the dental crown and part of the dental neck. The most thickness of the enamel
is on the apical surface of the tooth (
The enamel consists of the enamel prism. In longitudinal section the prism is S-shaped in transverse section it is polyhedral. The enamel prism fascicles state in relation to the dentin is rough perpendicular. The prism contains the organic fine fibril network and salt crystals. The glue substance between prisms is less mineralized than the prism.
On tooth longitudinal section the lines of Retzius are seen. The lines are the spaces, which are weakly mineralized. On the longitudinal section light and dark strips are also seen because one part of the prism is cut transverse while the other part is cut longitudinally.
In the enamel the spindles, fascicles, and lamellae are seen.
The enamel spindles are the dentin tubules entering the enamel. Through the spindles the nutrient passes from the dentin tubules to the enamel.
The enamel fascicles are weakly mineralized projections entering the enamel from the dentine-enamel junction.
The enamel lamellae are weakly mineralized projections extending the enamel surface from the dentine-enamel junction.
The enamel fascicles and lamellae are harmful structures because bacteria and their poisons enter the structures so the enamel undergoes destroying.
The strength and
chemical structure of the enamel depend on the metabolism. Through the enamel
the water, ions, amino acids, glucose, and others substances can pass. The
substances enter the enamel from the saliva. The saliva influences the
permeability of the enamel. The enamel permeability becomes high if the acids,
parathormone, and spirit influence the enamel. The need of calcium, phosphor,
and fluorine causes enamel permeability.
The Dental Cuticle
It is thin organic lamina covering the enamel. The cuticle is derived from the stellate reticulum of the enamel (dental) organ. The cuticle on the teeth apical part is rapidly rubbed. It only remains on the tooth side surface. The cuticle protects the enamel from various harmful chemical substances.
The Dentine Structure
The dentin (Fig. 128) is a hard substance, which is permeated by numerous fine canaliculi called the dental tubules. The dentine includes 28% organic substances (predominantly collagen) and 72% of salts (calcium phosphate, magnesium phosphate, and calcium fluorine). The dentine has 2 layers: 1) the external layer called the mantle layer, in which the collagen fibers pass radially, and 2) internal layer called the juxtapulpar layer, in which collagen fibers run longitudinally.
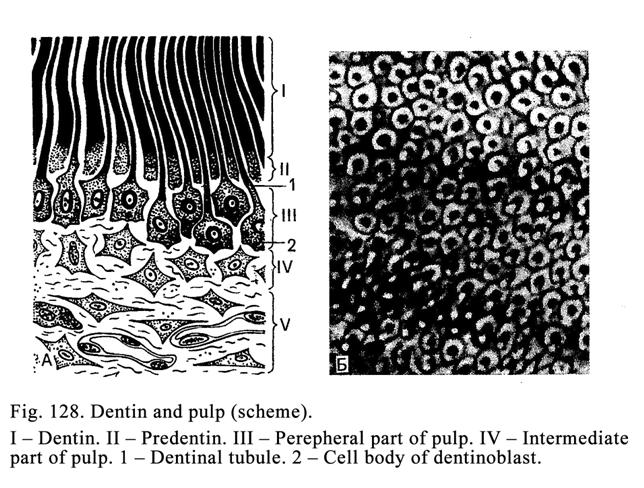
In the dentine peripheral part of the crown, neck, and root there are non-mineralized spaces. In the dental crown the spaces are large. They are called the interglobular spaces. In the dental root the spaces are small. They are called the granular layer of tooth root. The interglobular spaces take part in the dentine metabolism. The internal part of the juxtapulpar dentine is also not mineralized.
Dental tubules are fine canaliculi that pass radially from the pulp cavity towards the enamel (or towards the cement). Some dental tubules extend into the enamel (enamel spindles). In the juxtapulpar dentine there are 70000 dental tubules in 1 mm2 in the mantle dentine there are only15000-30000 tubules. In the dental crown the dental tubules branch weakly while in the root dentine they branch strongly.
The dental tubule walls are surrounded by circularly collagen fibers (peritubular dentine). The peritubular dentine is more mineralized, than inter tubular one (dentine between tubules).
Within the dentinal tubules there are the peripheral dentinoblast processes and fluid. The dentinal tubules take part in the dentine metabolism and provide it with the nutrients.
In the dentine there are weakly mineralized strips called the dental incremental lines. In the tooth longitudinal section the lines have longitudinal direction in the transverse section they are ring-like.
The dentine surface, which is attached to the enamel, contains numerous short elevations. Due to those the enamel connects to the dentine strongly.
The secondary dentine is formed after the tooth eruption. In case the external surface of the dentine is destroyed the dentinoblasts produce new dentine on the pulp cavity wall. That is also secondary dentine. The secondary dentine may be formed by dentinoblasts within the pulp cavity if the pulp is inflammatory. The secondary dentine is different from the primary one. For example, in the secondary dentine the directions of the dentinal tubules and collagen fibers are irregular; the number of the interglobular spaces in the secondary dentine is more, than in the primary dentine.
The Cement
The cement covers the dental neck and root. It contains 30% organic substances and 70% of salts (predominantly calcium phosphate and calcium carbonate). There are 2 types of the cement: 1) cellular cement and 2) acellular cement. The former covers the dental root apex the latter covers the dental neck and upper part of the root.
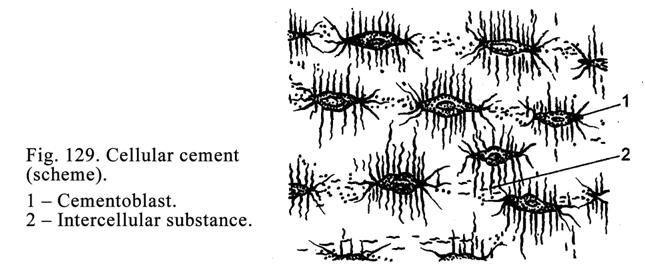
The cellular (Fig.129) cement may be regarded as the reticulofibrous bone. This cement contains branching cells, called cementocytes, and collagen fibers running in various directions. The cementocytes localize in lacunae, giving off number of canaliculi, which anastomose with those of other lacunae or with dental tubules providing the metabolism between the cement and dentine. In cement canaliculi there are the cementocyte processes.
Acellular cement contains glue substances and collagen fibers running in
longitudinal and radial directions. The internal ends of radial fibers extend
into the dentine while their peripheral ends extend into the socket wall of the
jaw to form the circular dental (gingival) ligament.
The Dental Pulp
The dental pulp localizes in the dental coronal pulp cavity and the dental root canal. The pulp is a variety of loose connective tissue. The dental pulp consists of 3 layers: 1) the peripheral layer attaching to the wall of the pulp cavity, 2) the intermediate layer, and 3) the central layer.
The peripheral layer (Fig. 128 III) consists of dentinoblasts, having processes. The cell length is 20-25 mm and thickness 6 mm. The dentinoblast cytoplasm contains Golgi complex, rough ER, and mitochondria. The peripheral processes of the dentinoblasts extend into dentinal tubules. They take part in dentinal metabolism and they have the ability of pain. It is confirmed by the presence of acetylcholine esterase in their ends. The intercellular substance of the peripheral layer of the dental pulp part contains collagen fibers, glycosaminoglycans, glycoproteins, proteoglycans, and others substances.
The intermediate layer (Fig. 128 IV) consists of non-maturated collagen fibers and non-differentiated cells, which can convert into dentinoblasts.
The central
layer of the dental pulp contains fibroblasts,
macrophages, adventitial cells, and collagen and reticular fibers. The dental
pulp provides the nutrient function.
The Periodontium
The periodontium consists of the fibrous tissue. It belongs to the supporting apparatus of the tooth. The periodontium contains collagen fibers. The internal ends of the fibers extend into the dental cement of the dental neck and root while peripheral ends extend into the jawbone. In the periodontium there is some loose connective tissue, in which blood vessels are present.
In the dental neck region collagen fibers form circular dental ligament while the dental neck and root are surrounded by the dento-alveolar ligament (periodontal ligament). The periodontium provides 2 functions: 1) it holds the dental root in the socket, and 2) it take part in nutrition of the teeth due to the blood vessels are present in the periodontal loose connective tissue.
The periodontium, gum, and socket wall constitute the special supporting apparatus.
The Eruption of the Baby Teeth
The baby teeth
erupt at 6-7th month of child. Before the eruption in the dental
crown the dental pulp accumulates and the pulp pressure becomes high. The
pressure influences the dental crown so the latter moves to the gum surface and
erupts. At first the cutting teeth erupt, then the canine teeth, then the cheek
teeth.
The Dental Root Development
If the tooth has one root (the cutting tooth and canine tooth), the lower margin of the enamel organ extends into the subjacent mesenchyme to form the epithelial tube. The peripheral mesenchymal cells, attaching to internal aspect of the tube, convert into the dentinoblasts, producing the root dentine. The central mesenchymal cells within the epithelial tube convert into fibroblasts, which produce the dental pulp in the dental root canal.
If the tooth has two roots (premolar), from the lower part of the enamel organ margin two processes arise towards one onother. At last the processes join to form two apertures. From every aperture the epithelial tube extends into the subjacent mesenchyme. Within the tubules the dental roots are formed.
If the tooth has three roots (cheek tooth), from the lower part of the enamel organ margin three processes arise towards one onother. When the processes join, three apertures are formed. From the apertures the epithelial tubes go into the mesenchyme. Within every tube the dental root is formed.
The Dental Cement Development
The mesenchymal cells of the dental saccule internal layer differentiate into cementoblasts, which destroy the epithelial tube wall covering the root. After it the cementoblasts come in contact with the dentine of the dental neck and root so the cementoblasts become active and produce the cement (collagen, glycosaminoglycans, proteoglycans, and others substances). At first they deposit the cement on dental neck surface then move towards the apical part of the dental root. Here the cementoblasts are concluded themselves by the cement and converted into cementocytes locating in lacunae to form the cellular cement. The dental cement of the dental neck and the initial part of the dental root does not contain the cementocytes therefore it is called the acellular cement.
The Periodontium Development
The periodontium is derived from mesenchymal cells of the dental saccule external layer. These cells undergo differentiation into the fibroblasts producing collagen and other components of the connective tissue of the intercellular substance.
Eruption of Permanent Teeth
At the fifth month of the embryonic life the permanent cutting teeth germs and the first permanent cheek tooth germ develops, after it the premolar germs and canine tooth germs form. The germs of the second cheek teeth develop at the first year of the postnatal life. The germs of the wisdom teeth form at the fifth year of the postnatal life. At first the permanent cutting teeth erupt (at the 6-7th year of the postnatal life).
All permanent teeth except cheek teeth have their own baby tooth precursors (precursors of permanent cutting teeth are baby cutting teeth, precursors of permanent canine teeth are baby canine teeth, and precursors of permanent premolars are baby molars).
The permanent tooth germs having baby tooth precursors form along with those and localize with them in the same cavity. Later the permanent tooth germ is separated from precursor tooth germ by the bone lamina.
When dental lamina is longer and longer the molar (cheek) tooth germs are formed (they have not baby precursors).
Permanent Teeth, Having Baby Precursors,
Eruption
Within the permanent tooth germ crown the pulp mass enlarges to press up the crown. At the same time osteoclasts destroy the bone lamina separating permanent tooth germ from the baby tooth root. After it the crown approaches to the baby tooth root. At the same time the osteoclasts destroy the dentine and cement of the baby tooth root. When the permanent tooth crown reaches the gum surface the entire baby tooth root is destroyed. Therefore the baby tooth crown falls away and the permanent tooth crown erupts.
Eruption of the Permanent Cheek Teeth
They erupt similar
to the baby teeth. The first cheek tooth erupts at 7th year of the
postnatal life. The second that erupts at 13th year and third cheek
tooth erupts at 24th year after birth.
The Tooth Blood Supply
The jaw artery branch enters the pulp cavity of tooth through the root apical foramen, where it undergoes ramification to form the capillary network. The capillary vessels join to form venules and veins. The veins leave the tooth cavity through the same apical foramen. In the pulp there are lymphatic capillary and small lymphatic vessels. The temperature and mechanical pressure at the time of chewing influence the blood flow in the pulp vessels.
He Tooth Nerve Supply
The trigeminal nerve branch enters the cavity of tooth through the apical foramen. The nerve fibers of the branch end by the receptors. Some nerve fibers enter the dental tubule initial parts (pain reception). In the pulp there are non-myelinated nerve fibers ending in the pulp blood vessels.
Age Dependent Changes of Teeth
In an old person the enamel and dentine of the tooth crown apical part are rubbed. The enamel becomes muddy. On the enamel cracks are seen. On its surface the salts deposit. The enamel, dentine, and cement contain less organic substances while the quantity of the mineral substances increases so the permeability of the enamel, dentine, and cement becomes low. In an old person the new dentine does not form, while the cement grows. The dental pulp undergoes atrophy because its blood vessels undergo sclerosis. It leads to decreasing of the dental tissue nutrition. The quantity of the dentinoblasts decreases.
The Oesophagus
The oesophagus is the tube joining the pharynx with the stomach.
The Oesophagus Development
The esophagus epithelium is derived from the ectoderm while the connective tissue and smooth muscle are derived from the mesenchyme. The esophagus peritoneum mesothelium is derived from the mesoderm.
The Oesophagus Structure
The oesophagus wall (Fig. 130) includes 4 tunics: 1) mucosal layer, 2) submucosal layer, 3) muscular tunic, and 4) adventitial layer (above the diaphragm) or peritoneum (lower the diaphragm).
The mucosal along with submucosal layers form longitudinal folds. The mucosal layer includes 3 laminae: 1) epithelium, 2) proper mucosal lamina, and 3) muscular mucosal lamina.
The epithelial lamina consists of stratified squamous non-keratinized (sometimes keratinized) epithelium, in which there are 3 layers: basal layer, middle layer and superfitial layer.
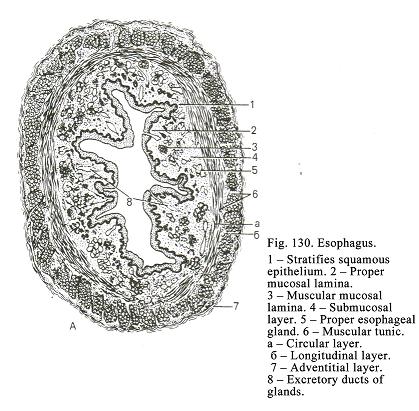
The epithelium thickness is about 500 mm. It includes 20-25 cell thickness. In old persons the epithelium may be keratinized.
The proper mucosal lamina consists of loose connective tissue containing lymph nodes and terminal regions of the esophageal cardiac glands. The part of the glands localizes between the thyroid cartilage and 5th tracheal cartilage. The other part of those is near the stomach. These are simple tubular glands. Cuboidal mucous epithelial cells line their terminal regions. Among these cells there are endocrine cells. The cardiac glands release the mucous secretion, which help to move the food through the esophagus. In places, where there are some cardiac glands, extensions, ulcers, and tumors may be formed.
The muscular mucosal lamina is made up of thin longitudinal bundles of smooth muscle cells. The thin muscular bundles are seen opposite the cricoid cartilage. Near the stomach the bundles become thick. Here the thickness of the muscular mucosal lamina is about 300 mm. The muscular mucosal lamina contraction promotes to move the food through esophagus.
The submucosal layer consists of loose connective tissue containing proper esophageal glands. These are composed compound tubular-alveolar glands. Mucous epithelial cells line the gland terminal regions. From the terminal regions small ducts, lined by the simple cuboidal epithelium, arise. These ducts enter the larger ducts lined by stratified squamous non-keratinized epithelium. They pass through the muscular mucosal lamina and reach the proper mucosal lamina. Here the gland ducts becomes wide to form ampullae. The gland mucous secretion moistens the food to promote its passing through the esophagus.
The muscular tunic consists of 2 layers: 1) internal circular layer and 2) external longitudinal layer. Between the layers there is the connective tissue. The esophagus upper one third is made up of the striated muscle, the middle third includes striated and smooth muscle, and lower third consists of smooth muscle only. Between internal and external muscle layers there is some loose connective tissue. Due to the internal muscle layer 2 sphincters are formed. The upper sphincter is opposite the cricoid cartilage while lower one is near the stomach.
The adventitial layer consists of loose connective tissue connected with that of the muscle tunic and mediastinum space. The serosal layer, consisting of loose connective tissue, lining by mesothelium, covers the sub-diaphragmatic esophagus part.
The Oesophagus Blood Supply
In the submucosal layer there are 2 arterial plexuses and in the mucosal layer there is 1 arterial plexus. Arterioles of the plexuses branch to form capillary network, localized deep to the proper mucosal lamina. The capillary vessels surround the esophageal glands and proper esophageal glands. The capillaries drain into venules, which join to form veins. The latter enter the submucosal venous plexus. In the esophagus wall there are lymphatic capillary vessels, submucosal lymphatic vessels, inter-muscular lymphatic vessels, and (sometimes) subserosal lymphatic vessels.
The Oesophagus Nerve Supply
In the oesophagus wall there are intrinsic neural plexuses: 1) adventitial neural plexus, 2) sub-adventitial neural plexus lying on the muscular tunic surface, 3) inter-muscular neural plexus, and 4) submucosal neural plexus.
The sensitive (afferent) nerve fibers are dendrites of the spinal ganglion neurons and dendrites Dogels II type neurons. The sensitive nerve terminals end with various type receptors (bush-like receptors and encapsulated nerve corpuscles). The receptors are seen in all layers and laminae of the esophagus wall and its epithelium (free endings).
The efferent (sympathetic and parasympathetic) nerve fibres are axons of the sympathetic nerve ganglion neurons and of the intrinsic nervous plexus neurons (Dogels I type neurons). These nerve fibers end with neuromuscular terminals or neurosecretory terminals.
Due to the neurosensory neurons (Dogels II type neurons) and efferent or motor neurons (Dogels I type neurons) the local reflex acres are formed. Due to the acres the rough thick cluster of food or sharp thing can pass through the esophagus. When the movement of a rough thing influences the esophagus receptors, the nerve impulse is provided. The impulse travels through the sensory neuron to motor neuron, which transmits the impulse to the smooth muscle. It leads to the relaxation of the muscles and results in the further movement of the thing.
On the histology preparation the contact between the esophagus epithelium and stomach epithelium are seen. In the proper mucosal lamina of the esophagus and stomach there are cardiac glands.
CHAPTER
21
THE
STOMACH & THE SMALL INTESTINE
The stomach and the intestine belong to the intermediate part of the alimentary system.
The Stomach
The Stomach Development
The development of the stomach begins at the 4th week of the embryonic life. Before the end of the second month all the stomach parts are fully developed. Its epithelial lining is derived from the endoderm, the smooth muscle and connective tissue are derived from mesenchyme, and mesothelium of the serosal lamina is derived from the mesoderm.
The Stomach Structure
The stomach wall consists of 4 layers: 1) mucosal layer, 2) submucosal layer, 3) muscular tunic, and 4) serosal layer (Fig. 131).
The Mucosal Layer
The mucosal layer includes 3 laminae: 1) epithelium, 2) proper mucosal lamina, and 3) muscular mucosal lamina.
On the mucosal layer surface there are gastric fields, gastric pits, and gastric folds.
The gastric
fields are places separated from each other by
veins present in the connective tissue surrounding the gastric gland clusters.
The fields are 1-
The gastric pits are the epithelium deep depressions into the proper mucosal lamina. These pits extend for a variable distance into the thickness of the proper mucosal lamina. The pit depth in the pylorus is ½ of the proper mucosal lamina thickness in the other parts of the stomach their depth is ¼ of the proper mucosal lamina thickness.
Both mucosal and submucosal layers form the gastric folds.
The epithelial lamina (epithelium) of the mucosal layer is simple columnar mucous epithelium (superficial epithelium). The columnar cell basal part (end) lies on the basement membrane. In the basal part there is an oval nucleus. The slightly stained cell cytoplasm contains Golgi complex, smooth ER, and mitochondria. In the cell apical part there are mucous granules. The gastric superficial epithelium discharges mucous secretion, which protect the mucosal layer from chemical and mechanical actions.
The proper mucosal lamina consists of the loose connective tissue including gastric glands, solitary lymph nodules, nerve fibers, both blood and lymphatic vessels.
Gastric Glands
The gastric glands are divided into 1) cardiac glands, 2) gastric proper glands, and 3) pyloric glands. The gastric glands belong to simple tubular glands. Their quantity is about 42 millions. The gland ducts open into gastric pits. Every gland includes the isthmus, cervix, corpus, and fundus. Both the corpus and fundus constitute the terminal region of the gland.
Gastric Proper Glands
The gland (Fig.
132) is
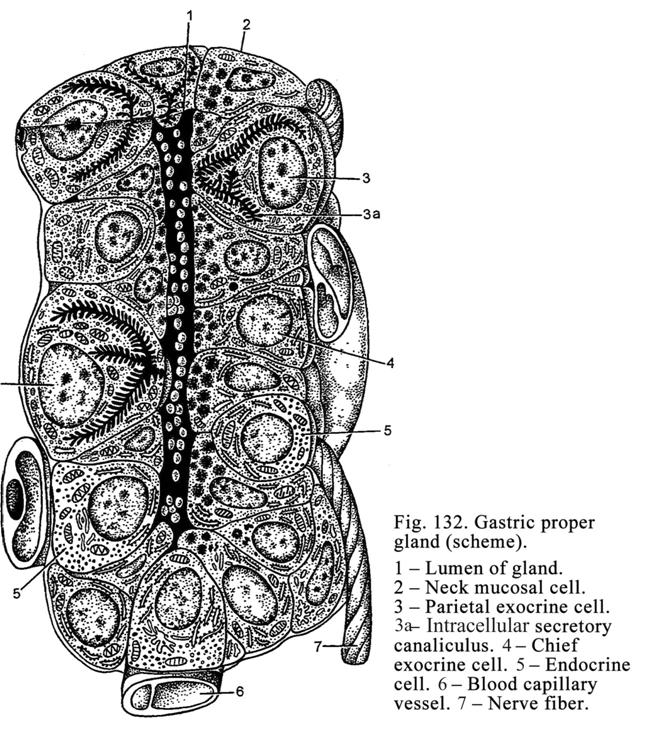
Chief exocrine cells, located in the corpus and fundus of a gland, are column shaped having slightly stained basophilic cytoplasm. Their round or oval nuclei localize in the cell center their apical part bears microvilli. The cell cytoplasm contains prominent rough ER, Golgi complex, and mitochondria. The cell apical part contains large (0.9-1 mm in diameter) secretory granules. Between the cells there are intercellular canaliculi. The chief exocrine cells release the pepsinogen (precursor of the pepsin), which is converted into pepsin in the acid environment. These cells also release the enzyme chymotrypsin influencing the milk in children.
The mucous exocrine cells are located in the corpus of glands. The cytoplasm is slightly stained. The cell apical parts bear microvilli. The flat nucleus of the cell lies in its basal part. In the cell cytoplasm there are prominent Golgi complex, smooth ER, and mitochondria. In the apical part of the cells mucous granules are seen. Between cells there are intercellular canaliculi. The cells release the mucous secretion.
The neck mucous cells (non-differentiated cells) are able to divide by mitosis. The cells localize in the gland neck. The neck mucous cells are column shaped their slightly stained cytoplasm contains the flat nucleus, located in the basal part of cells, Golgi complex, smooth ER, and mitochondria. In the cell apical part the mucous secretory granules are present. These cells release mucous secretion and replace the cells, which are destroyed.
The parietal exocrine cells apply to basal part of the chief and mucous cells. They are large ovoid polyhedral cells containing one or two large nuclei in their center. Their cytoplasm is strongly stained by acid dyes. In the cell cytoplasm there are thin canaliculi, through which ions of chlorine pass into the intercellular space (between the chief and mucous exocrine cells) and reach the gland lumen. The parietal exocrine cells release the chlorine and hydrogen and as a result the hydrochloric acid is formed.
The endocrine cells are in the gastric proper glands, pyloric glands, and cardiac glands. They localize between the exocrine cells (chief cells, mucous cells, and neck mucous cells).
The pyloric glands localize in the stomach pyloric part. The quantity of the glands is about 3.5 millions. These glands differ from the gastric proper glands in because 1) they are placed sparsely, 2) they have more branches, 3) they have a wide lumen, 4) they are shorter than gastric proper glands or cardiac glands, and 5) they do not contain the chief cells and parietal cells (chief and parietal cells are sometimes present). The most gland cells are mucous cells. Non-differentiated cells are also present. The pyloric glands produce the mucous alkaline secretion containing enzymes
splitting dipeptides.
Cardiac glands are located in the stomach cardiac part. Their quantity is 3.5 millions. They branch vastly. Mucous cells having slightly stained cytoplasm line the gland lumen. The flattened nucleus lies in the cell basal part; in the cell apical part the mucous granules are seen. In the cell cytoplasm there are Golgi complex, smooth ER, and mitochondria. The cardiac glands also include non-differentiated and endocrine cells. Some parietal and chief cells may be also present. The cardiac glands release mucous secretion containing enzymes, which are able to split dipeptides.
The Stomach Endocrine Cells
These are EC-cells, G-cells, ECL-cells, P-cells, D-cells, D1-cells, A-cells, and X-cells.
EC-cells release the serotonin and melatonin. The serotonin stimulates releasing of the enzymes and mucus from other cells it also influences the stomach musculature contractions. The melatonin regulates the gut function depending on the time of the day.
G-cells are located predominantly in pyloric and cardiac glands they release gastrin and gastric encephalin. The gastrin stimulates the stomach muscle contraction,
the pepsin and hydrochloric acid releasing from chief and oxyntic cells respectively. The gastric encephalin is the neurotransmitter of the pain. The nerve cell receptors catch the encephalin therefore the neurons do not perceive the pain impulses. But nicotine, alcohol and drug molecules replace the encephalin easily. If the person smokes, takes alcohol or drugs, the gastric encephalin becomes less and less and as a result the person gets used to the nicotine, alcohol, or drug.
ECL-cells release histamine stimulating the hydrochloric acid production from the parietal cells.
P-cells produce substances, which stimulate the release of the hydrochloric acid from the parietal cells, enzymes from the pancreas, and stimulate the gallbladder muscle contraction.
D-cells predominantly localize in the pyloric glands, produce somatostatin inhibiting the protein enzymes synthesized in others gland cells.
D1-cells are predominantly present in the pyloric glands produce vasoactive intestinal polypeptide (VIP), which reduces the blood pressure and stimulates the pancreas function.
A-cells produce the enteroglucagon (hormone splitting the glycogen in the liver and muscles and as a result the blood sugar level becomes high).
X-cell function is unknown.
The fundus of the gastric glands lies on the muscular mucosal lamina.
The muscular mucosal lamina consists of 3 layers of the smooth muscle cells: internal circular, middle longitudinal, and external circular muscle cells. The part of the smooth muscle cells migrates into the proper mucosal lamina. The muscular mucosal lamina provides the motility of the mucosal layer and releasing of the gastric gland secretions.
The Submucosal Layer
It is made up of the loose connective tissue containing a number of reticulr fibers. In the submucosal layer there are nervous, arterial, venous, and lymphatic plexuses.
The Muscular Tunic
The tunic is
slightly developed in the gastric cardiac part, better in the gastric fundus
and corpus, and the best in the gastric pyloric part. The muscular tunic
consists of 3 layers: 1) external longitudinal layer, which is the continuation
of the esophagus, 2) middle circular layer, due to which the pyloric sphincter
is formed (its thickness is about 3-
The Serosal Layer
It consists of the loose connective tissue covered by mesothelium.
The Stomach Blood Supply
The gastric arteries pass across the serosal layer and muscular tunic to form the submucosal arterial plexus. The arterial plexus branches enter the mucosal layer to form the mucosal arterial plexus, from which the arterioles arise. The arterioles branch to form the capillary network. The capillaries run into the venules and star-like veins having thin walls. The veins lie near the epithelium lining. If the epithelium is damaged, the star-like vein wall is ruptured and the gastric bleeding begins. The venules run into the mucosal venous plexus, from which blood enters the submucosal venous plexus. The veins of the mucosal and submucosal plexuses have valves.
The lymphatic capillaries run into lymphatic vessels forming the submucosal lymphatic plexus. From there the lymph flows into intramuscular lymphatic plexus.
The Stomach Nerve Supply
Three neural plexuses provide the nerve supply of the stomach: 1) submucosal neural plexus, 2) intramuscular neural plexus, and 3) subserosal neural plexus.
The plexuses include sympathetic and parasympathetic nerve fibres and afferent (sensitive) nerve fibers.
The sympathetic nerve fibers are axons of efferent neurons of the sympathetic nervous ganglions. The parasympathetic nerve fibres are axons of efferent neurons (Dogels cell I type) of the parasympathetic nervous ganglions. In the stomach pyloric part there is a number of the sensitive neurons (Dogels cell II type), which dendrites end by chemoreceptors within the duodenum. The chemoreceptors are sensitive to the hydrochloric acid. The Dogels cell I types and Dogels cell II types constitute the local arcs regulating the evacuation of the acid substance from the stomach into the duodenum. When the acid stomach contents enter the duodenum, chemoreceptors send impulses through the local arcs to the pyloric sphincter and a result the sphincter closes. When the acid contents in the duodenum become alkaline, the chemoreceptors cannot send the impulses to the pyloric sphincter and it opens. It leads to pass the next portion of the acid contents from the stomach into the duodenum.
Afferent nervous fibers, ending with receptors, are dendrites of the neurons, localizing in the spinal sensory ganglion. When the sympathetic nervous fibers are stimulated, the motor and secretory functions of the stomach are inhibited. If the parasympathetic neuron fibers are stimulated, the motor and secretory functions of the stomach are activated.
The stomach provides 1) secretory function, 2) mechanical function (evacuating the stomach contents from the stomach into the duodenum), 3) resorption function, 4) excretory function, 5) endocrine function, 6) anemia prevention function, (production of the intrinsic factor, which promotes the erythrocytes formation),
The secretory function is characterized by releasing gastric enzymes (pepsin, chymotrypsin, and lipase). The pepsin splits proteins. The pepsin is produced by the action of hydrochloric acid on pepsinogen (precursor of pepsin). The chymotrypsin is present in children (it splits the milk). The lipase splits lipids of the milk in children.
The anemia prevention function is provided by the intrinsic factor, which combines with vitamin B12 (present in ingested food and constituting the extrinsic factor) to form the complex necessary for normal formation of erythrocytes.
Through the gastric wall the water, spirit, salts, and glucose are absorbed.
Through the wall of the stomach the ammonia, urea, and alcohol splitted components excrete into the stomach lumen.
The stomach endocrine cells release serotonin, gastric encephalin, vasoactive intestinal polypeptide, hormone splitting the glycogen, and others hormones. The hormone actions are either local at the neighboring cells or at the cells at the distant place (through the blood stream).
The Small Intestine
The small intestine (Fig. 133) consists of 3 parts: 1) the duodenum, 2) the jejunum, and 3) the ileum.
The small intestine wall includes 4 layers: 1) the mucosal layer, 2) the submucosal layer, 3) the muscular tunic, and 4) the serosal layer.
The small intestine epithelium is derived from endoderm, the smooth muscle and loose connective tissue are derived from the mesenchyme, and mesothelium of the serosal layer is derived from mesoderm.
The Mucosal Layer
The relief of the small intestine mucosal layer is made up of folds, villi, and crypts.
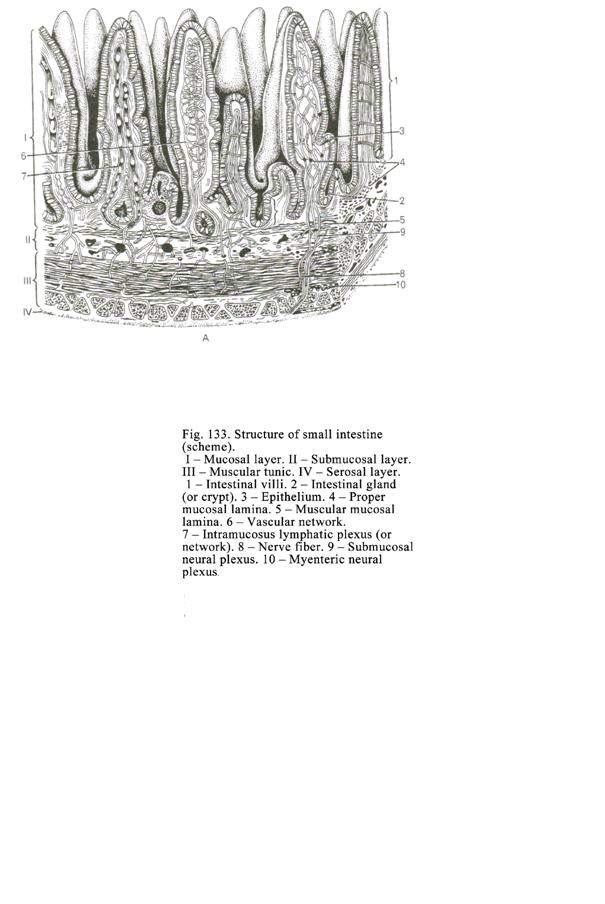
The mucosal and submucosal layers form the folds of the small intestine. The folds are circular direction.
The villi (Fig. 133. 1) are the projections of the mucosal layer loose
connective tissue covered with the columnar epithelium. Within the villi there
are smooth muscle cells, arteriole, blood capillary vessels, lymphatic
capillary vessels, and venules. In the duodenum the length of the villi is
about 0.3-
The epithelium, covering the villi, is called the columnar epithelium. The epithelium includes 4 types of cells: 1) the columnar epithelial cells with a striated border, 2) M-cells, 3) goblet cells, and 4) endocrine cells.
The columnar epithelial cells with striated border bear microvilli on their apical surface. The length of the microvilli is about 1 mm, their diameter is about 0.1 mm; the distance between the microvilli is 0.01-0.02 mm. Among microvilli there are the alkaline sulphatase, peptidases, lipases and others enzymes. The microvilli contain the microfilaments and microtubules. Due to the microstructures the absorption of the food and movement of the microvilli are provided. The surface of the microvilli is covered with glycocalyx. The digestion on the striated border surface is called the digestion on the intestine wall surface. In the cell cytoplasm there are prominent rough ER, Golgi complex, mitochondria, multivesicular bodies (within the vesicles there are some small vesicles), and microfilaments, forming the cortical layer in the cell apex part. Active oval nucleus localizes in the cell basal part. The cells join by tight intercellular junctions (zonula occludens), desmosomes, and adhesive belts. In the cell membrane lateral surface there are Na-ATPase and K-ATPase. The columnar epithelial cells with striated border 1) produce digestive enzymes, 2) take part in digestion, and 3) the food absorb.
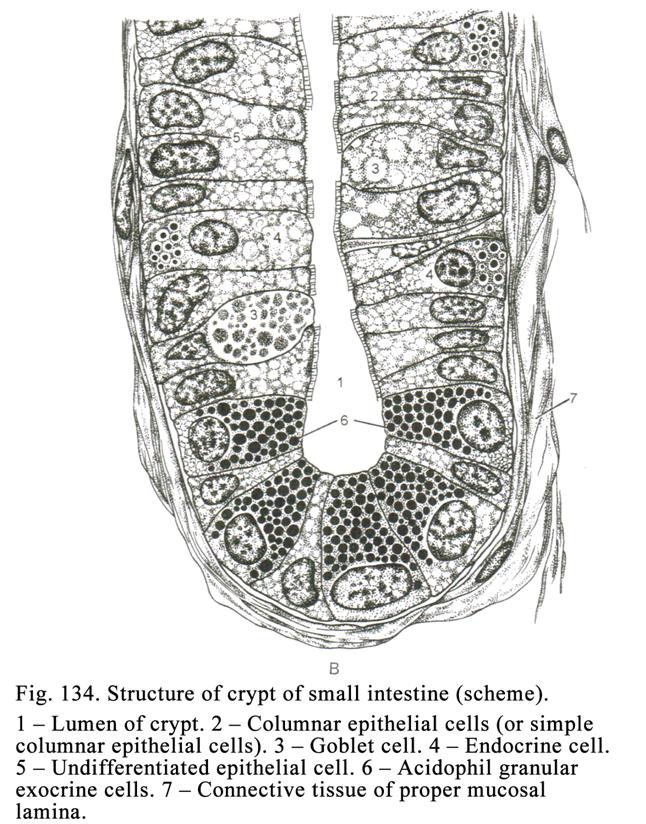
The M-cells localize in the place, where the mucous layer lymph nodules are present. The cell membrane of epical surface has folds. The folds trap antigens and send them to the lymph nodules so the antigens come in contact with lymphocytes and stimulate their differentiation.
The goblet cells are mucous cells. They contain smooth ER, Golgi complex, mitochondria, and flattened nucleus localized in the cell basal part. On the smooth ER the mucus is synthesized. The mucous secretory granules accumulate in the cell apical part, so the apical part becomes wide therefore the cell assume the goblet appearance. After the secretion release the cell becomes column shaped. In the small intestine villi and crypts there are 7 types of endocrine cells.
The Crypts
The crypts (Fig.
134) are simple tubular glands localizing in the proper mucosal lamina. The
crypt length is about
The acidophil granular exocrine cells localize in the gland body or gland fundus. The cells contain prominent Golgi complex, rough ER, mitochondria, and round nucleus. In the cell apical part there are acidophil granules containing glycoproteins. The cell cytoplasm contains zinc and enzymes (peptidase, acid phosphatase, and others enzymes), lysozyme killing the bacteria. The acidophil granular exocrine cells split proteins, kill bacteria, and neutralize the hydrochloric acid.
The crypts and villi constitute the complex due to 1) their anatomical proximity (the crypts open between the villi), 2) the crypt cells produce enzymes splitting the food on the surface of villi, and 3) undifferentiated cell of crypts replace the crypt cells and villus cells which die in 5-6 days.
Intestine Endocrine Cells
In the small intestine there are 7 types of endocrine cells: 1) EC-cells release secretion motilin and substance P, 2) A-cells produce hormone (glucagon) splitting the glycogen, 3) S-cells release the secretin stimulating the pancreas function, 4) I-cells release cholecystokinin, stimulating the liver function, and pancreozymin, stimulating the pancreas function, 5) G-cells produce gastrin and encephalin, 6) D-cells release the somatostatin, and 7) D1-cells produce vasoactive intestinal polypeptide (VIP).
The Proper Mucosal Lamina
It is made up of
loose connective tissue containing a number of reticular fibers and reticular
cells. In addition the proper mucosal lamina contains solitary lymphatic
nodules (they are about
The Muscular Mucosal Lamina
It consists of 2 layers: 1) internal circular layer and 2) external longitudinal layer. Between the muscle layers there is a loose connective tissue thin layer.
The Submucosal Layer
It is made up of loose connective tissue containing nervous plexus, arterial and venous plexuses, and submucosal lymphatic layer. In the duodenum submucosal layer there are duodenal glands. Mucous cells of the glands having light cytoplasm and flattened nuclei predominantly line the gland terminal regions. The cell cytoplasm contains Golgi complex, smooth ER, and mitochondria. The cell apical part contains mucous secretory granules. In additional in the gland terminal region the goblet cells, undifferentiated cells, and parietal cells (sometimes) are seen. The cuboidal epithelium lines the duodenum gland small ducts while columnar epithelium lines large duct opening in the intestine lumen. The duodenum gland secretion is alkaline it contains peptidases. The secretion splits dipeptides and influences the acid substance, entering from stomach, so the substance becomes alkaline.
The Intestine Muscular Tunic
It consists of 2 layers of smooth muscle cells: 1) internal circular layer and 2) external longitudinal layer. Between the muscular layers there is loose connective tissue, where 2 nervous plexuses are present: 1) myenteric neural plexus, and 2) myenteric sensitive neural plexus. Due to the local contraction of the internal muscle layer the intestine contents are intermixed due to the simultaneous contraction of both circular and longitudinal muscle layers the intestine contents are pushed to its distal end.
The Serosal Layer
It consists of connective tissue covered by the mesothelium. The doubled serosal layer forms the mesentery joining with the abdomen cavity dorsal wall. The animal body has the horizontal position therefore the mesentery cannot turn to one or other direction. The human body has the vertical position therefore its mesentery is able to turn to right or left direction. It makes the food movement through the gut stop, its blood supply as a result the intestine wall falls in necrosis. In order to prevent the necrosis the human body must assume the horizontal position. It helps to restore the correct condition of the intestine.
The Intestine Blood Supply
There are three arterial plexuses 1) submucosal, 2) intramuscular, and 3) mucosal plexuses provide the intestine blood supply. The plexuses give off the arterioles, which branch to form blood capillary vessel networks in tunics and layers of the intestine wall.
The arterioles of the mucosal arteriolar plexus extend into the intestine villi and branch to form blood capillary vessels, which run into venules of the villi. The venules run into the mucosal venous plexus, from which the blood outflows into submucosal venous plexus.
The lymph outflows from the villus through the lymph capillary vessels into the larger lymph vessels then into submucosal lymphatic plexus.
The Intestine Wall Nerve Supply
Two nervous plexuses provide the innervation of the intestinal wall: 1) myenteric neural plexus and 2) myenteric sensitive neural plexus.
The myenteric neural plexus is represented by axons of the sympathetic node neurons and axons of the parasympathetic node neurons (I type Dogels cells). Efferent (sympathetic and parasympathetic) nerve fibers end with motor endings (neuromuscular terminals) on the smooth muscle and neurosecretory terminals on the glands (crypts).
The myenteric sensitive neural plexus consists of the spinal ganglion neuron dendrites, parasympathetic ganglion neuron (II type Dogels cell) dendrites, and vagal ganglion neuron dendrites. Thus, the intestine includes both sympathetic and parasympathetic reflex arcs. In the intestine there are not only autonomic reflex arcs, including the chain, containing 3 neurons, but also arcs containing 4 neurons. For example, the first arc neuron is in the vagal ganglion, the second neuron is in the vagal nucleus, the third neuron (II type Dogels cell) is in the parasympathetic (intramural) ganglion, and the fourth neuron (I type Dogels cell) is also in the intramural ganglion.
In the small intestine wall there are local reflex arcs. The arcs localize in the parasympacetic ganglions. The arcs include 2 neurons (sensitive neuron or II type Dogels cell and effector neuron or I type Dogels cell).
The Small Intestine Functions
The small intestine provides 1) the food breakdown, 2) absorption the food, 3) intermixing of the food, and 4) production of hormones (serotonin, secretin, cholecystokinin, and others hormones).
The chemical food breakdown 1) is provided by the pancreas enzymes in the central space of the small intestine, 2) is provided by the crypt enzymes on the intestinal wall surface, and 3) is provided by mucous epithelial complex enzymes along with the pancreas enzymes. What is the mucous epithelial complex? The epithelium of crypts and villi is replaced by the new epithelium in 5-6 days. The mature epithelium, divested from the villi and crypts, is the mucous epithelial complex.
The trypsin, peptidase and others enzymes split the proteins. The nuclease splits the nucleic acids. The amylase, maltase, lactase, and others enzymes split the carbohydrates. The lipase splits the lipids.
The striated border of the columnar cells, covering the intestine villi, absorbs the splitting products. Every time the villi contract and relax. When the absorption function of the intestine is active, the villi contract 4-6 times per minute. Smooth muscle cells, localizing in the stroma of the villi, provide contraction of the villi. The smooth muscle cells direct radially around the blood vessels and lymph capillary of the villus. The central ends of the muscle cells attach with connective tissue, surrounding the vessels, while their peripheral ends attach with the basal membrane of the epithelium.
When the smooth muscle cells contract, the villus becomes small, its connective stroma squeezes. The vessels, localizing in the villus center, do not squeeze. The alterations within the villus at the time of its contraction promote entering the food into the vessels.
When the smooth muscle cells relax, the villus volume becomes large, and the pressure within the villus becomes low. This promotes the food absorption into the villus.
Thus, the villus acts similar to the eyedropper. Per minute the intestine villi absorb about 40 ml of the food substance.
The amino acids of proteins are absorbed by the striated border. The absorption of the lipids happens in two ways. In the first way the lipids are split by lipase to form glycerin and fat acid on the surface of the villus. The glycerin is just absorbed while the fat acids undergo etherification and then are absorbed into villus cells. Within the cells the ether splits to form the free fat acids, which combine with the glycerin to form the small fat droplets (1mm). The droplets pass through the cell membrane and villus stroma into lymph capillary vessel. In the second way the lipids undergo the emulsion and combine with the proteins to form the droplets on the striated border surface. After it the droplets enter the cell cytoplasm and intercellular spaces, reach the villus stroma, and enter the lymph capillary vessel.
The mechanical function of the intestine (intermix of the food) is provided by the intestine muscular tunic contraction to push the intestine substance to the gut distal part.
The endocrine function (releasing of hormones) is provided by endocrine cells localizing in the crypts and on the surface of the villi.
CHAPTER
22
THE
MIDDLE AND POSTERIOR PARTS OF THE ALIMENTARY CANAL
Except the stomach, small and large intestines the alimentary system includes the liver and the pancreas.
The Large Intestine
It includes 3 parts: 1) the colon including ascending, transverse, and descending parts, 2) the sigmoid colon, and 3) the rectum.
The
The colon wall is made up of 4 layers: 1) mucosal layer, 2) submucosal layer, 3) muscular tunic, and 4) serosal layer (Fig. 135).
The mucosal layer epithelium is derived from the endoderm, the epithelium of anal canal end is derived from the ectoderm, the serosal layer epithelium is derived from the mesoderm, and loose connective tissue and smooth muscle of the alimentary canal are derived from the mesenchyme.
The mucosal
layer relief includes crescent-shaped folds and
crypts. The epithelial lining of the mucosal layer consists of columnar
epithelial cells extending into the proper mucosal lamina to form crypts (Fig.
135 Į) or
simple tubular glands. The crypt lenth is about
The crypts contain 5 cell types: 1) goblet cells (the most number of the crypt cells are goblet cells), 2) columnar epithelial cells with the striated border, 3) endocrine cells, 4) non-differentiated cells, and 5) acidophil granular exocrine cells.
The proper mucosal lamina consists of the loose connective tissue containing number of the reticular fibers and reticular-like cells. In the proper mucosal lamina there are a number of lymph nodes (more than in the small intestine).
The muscular mucosal lamina is made up of 2 layers: 1) internal circular layer and 2) external longitudinal layer. Between the layers there is the thin connective tissue lamina.
The submucosal layer consists of the loose connective tissue, where the nervous, arterial, and venous plexuses and lymphatic vessel layer are present.
The muscular tunic is made up of 2 layers: 1) internal circular layer and 2) external longitudinal layer. The external layer is formed from 3 longitudinal bands (taenia coli). Between the bands haustrations are seen.
The serosal layer consists of the loose connective tissue covered by mesothelium. In many situations the peritoneum forms pouch-like processes that are filled with fat. These yellow masses are called appendices epiploiceae. The large intestine provides 1) the feces mass formation; 2) the feces mass evacuation, 3) mucus production, 4) excretory function (into large intestine lumen the metabolism products as urea, uric acid, and metal salts are released).
The Rectum
The rectum has some particular features. It is divided into 2 parts: 1) the upper part and the 2) lower part.
The upper part includes 3 circular and 8-10 longitudinal folds formed from submucosal and mucosal layers. The epithelial lining of the mucous membrane of the upper part wall is similar to that of the colon.
The lover part includes 3 zones: 1) anal columns, 2) intermediate zone, and 3) skin zone.
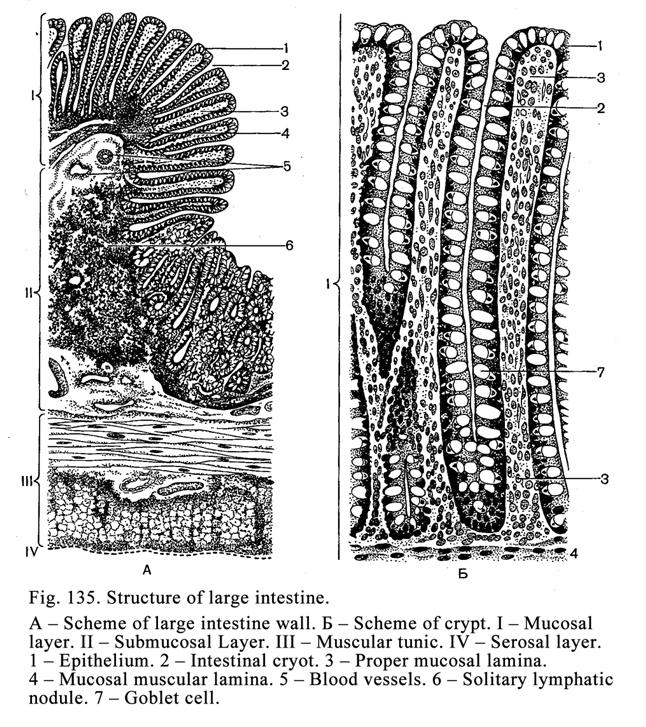
The cuboidal or columnar stratified epithelium lines the mucosal layer of the anal column zone. Among the cells endocrine cells are present. In the proper mucosal lamina there are wide sinusoid capillary vessels entering the hemorrhoid veins. Across the proper mucosal lamina the rudimentary rectal gland ducts pass. The muscular mucosal lamina is developed slightly or is absent.
The submucosal layer of the anal column zone consists of loose connective tissue. Here the rudimentary rectal gland terminal regions, arterial, venous, and nervous plexuses are present.
The muscular tunic of the anal column zone consists of the usual internal circular muscle layer and usual longitudinal muscular layer.
The adventitial layer of the anal column zone consists of loose connective tissue.
The Intermediate Zone
It is ring-shaped.
The intermediate zone wall width is about
The Skin Zone
Stratified squamous
keratinized epithelium lines the skin zone. In the zone there are some sweat
and sebaceous glands. In the distance of
The internal anal sphincter is formed from circular smooth muscle layer the external anal sphincter is formed from striated (skeletal) muscle.
The Large Intestine Blood Supply
In the large intestine wall there are submucosal arterial plexus and mucosal arterial plexus, giving off arterioles, branching in capillary vessels, which run into venules. The venules run into the mucosal and submucosal venous plexuses.
The Vermiform Appendix
It branches from the blind intestine. Its wall consists of 4 layers: 1) the mucosal layer, 2) the submucosal layer, 3) the muscular tunic, and 4) the serosal layer.
The mucosal layer includes the epithelial lamina, proper mucosal lamina, and muscular mucosal lamina. The epithelial lamina is made up of the simple columnar epithelium. The proper mucosal lamina consists of loose connective tissue. The muscular mucosal lamina is poorly developed.
The epithelium extends into the proper mucosal lamina to form crypts (simple tubular glands). The crypts are predominantly lined by the goblet cells. There are also columnar cells with the striated borders, undifferentiated cells, acidophil granular exocrine cells, and endocrine cells.
The proper mucosal lamina contains a number of lymph nodules, which may completely fill mucosal and submucosal laminae. The lymphocytes of the nodules can infiltrate not only the connective tissue but also the epithelium and reach the epithelium surface.
The submucosal layer is not in fact separated from the mucosal layer because the muscular mucosal lamina is poorly developed.
The muscular tunic consists of 2 layers: 1) internal circular layer and 2) external longitudinal layer, which are complete (taenia coli are not present).
The serosal tunic of the appendix forms the mesentery attaching to the abdominal cavity dorsal wall. The vermiform appendix provides 1) the blood formation function and 2) protective function.
The Liver
The liver includes right and left lobes. The connective tissue capsule, giving off thin connective tissue layers into the liver parenchyma, covers the liver.
The Liver Development
At the 5th week of the embryonic life the endoderm gut wall extends to form the hollow outgrowth. From the cranial part of the outgrowth the liver parenchyma and epithelium of the hepatic ducts are formed. From its caudal part the epithelium of the
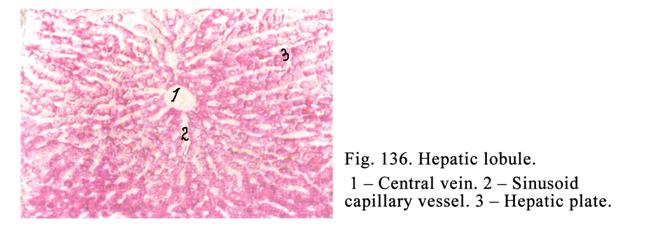
gallbladder and cystic duct are derived. From the outgrowth mouth the epithelium of the bile duct is derived. The connective tissue of the liver is formed from the mesenchyme.
Preliminary Remarks
The capsule and
peritoneum cover the liver. The peritoneum closely fuses with the capsule. The liver
parenchyma consists of 500000 hepatic lobules (Fig. 136). The lobule is a
structural and functional unit of the liver. They are prism shaped. The lobule
basal part is wide while its apical part is narrow. The lobule width is about
The simple lobules may fuse to form compound lobules. Thin connective tissue layers separate the human liver lobules from each other. Thick connective tissue layers separate the animal (pig) liver lobules from each other. If the liver is damaged by alcohol (it is called cirrhosis of the liver), the lobules become smaller the connective tissue around lobules becomes thicker.
In the interlobular connective tissue there are interlobular and circum-lobular ducts and blood vessels (arteries and veins) called the portal triad.
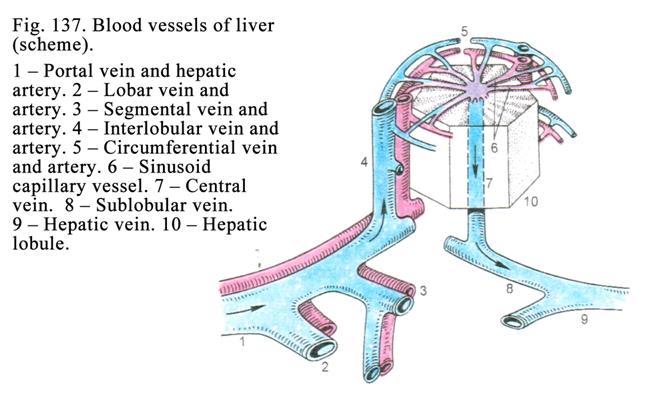
The Liver Blood Supply (Fig. 137)
The hepatic artery, carrying oxygenated blood, and portal vein, carrying blue blood, running from the alimentary tract, spleen, and some visceral organs, enter the liver hilus. Both the hepatic artery and portal vein are divided into 1) lobar vessels, 2) segmental vessels, 3) interlobular vessels, and 4) circum-lobular (surrounding lobules) vessels. The arterial capillary vessel runs from the circum-lobular artery while the vein capillary vessel runs from the circum-lobular vein. Both arterial and venous capillary vessels join to form the intralobular sinusoid capillary vessel. The latter runs into the central vein of the lobule running into the sub-lobular vein, which runs into the hepatic vein branch, the hepatic vein (3-4 hepatic veins) enters the vena cava inferior.
Thus, the liver blood supply includes 3 systems: 1) tributary system, 2) circulatory system, and 3) outflow (efflux) system. The initial part of the tributary system is the hepatic artery and portal vein; its end is the circum-lobular vessels. The interlobular sinusoid capillary vessels represent the circulatory system. The outflow system onset (initiation) is represented by the central vein; the efflux system end is represented by the hepatic veins.
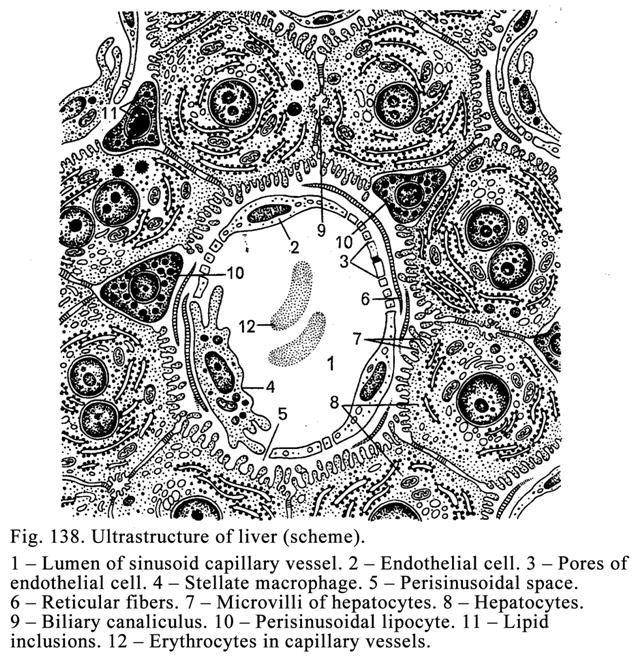
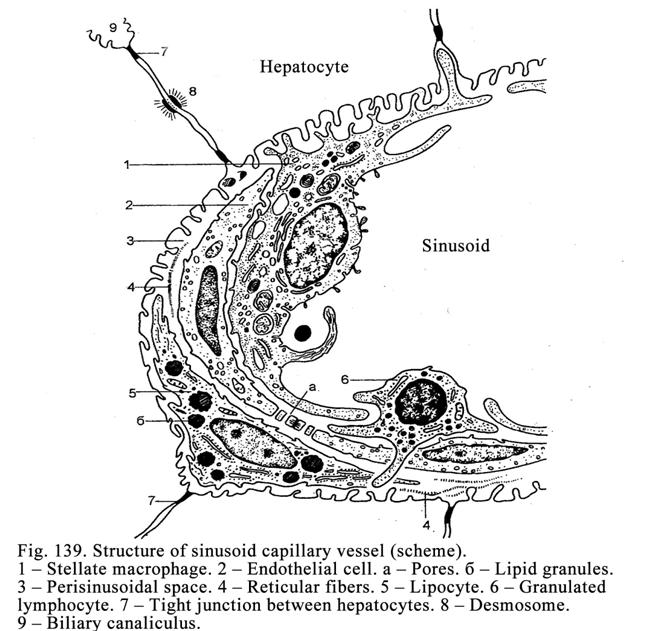
The interlobular and circum-lobular arteries are muscular artery types their branches have sphincters regulating the oxygenated blood influx. The interlobular and circum-lobular veins are vessels containing poorly developed muscle cells in their walls. Their branches have sphincters regulating the blue blood influx. The central and sub-lobular veins are trabecular vein types. The hepatic vein branches have sphincters regulating the blood outflow from the liver.
Along the periphery of the each lobule there are angular intervals filled with connective tissue. These intervals are called portal canals. The canals forming the connective tissue network permeate entire liver substance. Each canal contains the interlobular artery, interlobular vein, and interlobular biliary duct. These three structures collectively form the portal triad.
The Hepatic Lobule
It consists of hepatic plates (two cells thick). The cells, constituting the plates, are called the liver cells or hepatocytes. The spaces between plates the intralobular sinusoid capillary vessels (Fig. 138) occupy. Round the vessels the perisinusoidal spaces are seen. The plates and capillary vessels run from the center to periphery radially. Every lobule has the peripheral, intermediate, and central zone.
The interlobular sinusoid capillary vessels (Fig. 138 & Fig. 139) are about 30 mm in diameter their wall is lined with endothelial cells. In the endothelial cells (near the boundary between cells) there are pores to form the sieve. Among the endothelial cells the macrophages (stellate macrophages) are seen. The macrophages are derived from monocytes. They provide the protective function. Within the sinusoid capillary vessels there are pit-cells deriving from large lymphocytes. In their cytoplasm secretory granules are seen. The pit-cells kill damaged hepatocytes. When the liver cells get better, the pit-cells release factors stimulating the division of hepatocytes.
In the central and peripheral zones of the lobules the endothelial cells and macrophages lie on the basement membrane but in the intermediate zone the basement membrane is absent.
Perisinusoidal spaces (Fig. 138. 5) are 1mm in width. They surround interlobular sinusoid capillary vessels (Fig. 139). The spaces contain the fluid that is similar to the blood plasma. Here the macrophage processes, hepatocyte microvilli, argentophil fibers, surrounding hepatic plates, and processes of perisinusoidal adipocytes are present.
The perisinusoidal lipocytes (Fig. 138. 10) are 5-10 mm in diameter. They localize between hepatocytes. Their cytoplasm contains lipid droplets, number of ribosomes, and mitochondria. When the liver is damaged, the quantity of perisinusoidal lipocytes becomes increased. The perisinusoidal lipocytes 1) accumulate vitamins A, D, and others, and 2) produce argentophil fibers.
Hepatic plates consist of two rows of hepatocytes applying to each other. The denticulate intercellular junctions, desmosomes, and tight junctions join hepatocytes with each other. The hepatic plates associate with each other by anastomoses to form the network. The hepatic plates are similar to the exocrine gland terminal regions and similar to the endocrine gland cluster cells. On the one hand the hepatic plates synthesize and release the bile into the ducts on the other hand they release some products into the blood (glucose, proteins, and vitamins).
Biliary Canaliculi
Biliary canaliculi are within the hepatic plates. They do not have their own wall. Their wall is formed by hepatocytes. The hepatocytes give off microvilli into the biliary canaliculus lumen. The microvilli contain microfilaments providing movements of the microvilli. The microvillus movements provide the bile flow in the biliary canaliculi. If the microvilli do not move, the cholestasis occurs.
In the normal liver the biliary canaliculi is not communicated with the perisinusoidal spaces and interlobular sinusoids. When the liver undergoes some damage (hepatitis), the hepatocytes are destroyed and the biliary canaliculi get communicated with intralobular sinusoids (intralobular sinusoid capillary vessels). Through this communication the bile flows into the sinusoids and circulation and the bile stains tissues, organs, skin, and eye sclera in yellow colour.
The biliary canaliculus enters the circum-lobular bile duct.
Portal Lobules
The portal lobule
consists of three segments of the usual adjacent hepatic lobules to form triangle
shaped structure (portal lobule). In the portal lobule center there is the
portal triad. Angles of the portal lobule end opposite the central veins. In
the portal lobule the blood flows from its center (portal triad) to its
periphery (central veins).
The Portal Acinus
The portal acinus consists of two segments of the adjacent hepatic lobules. It is rhombus-shaped. The rhombus sharp angles end opposite the lobule central veins. One obtuse angle of the portal acinus lies opposite the portal triad. In the portal acinus the blood flows from the center (portal triad) to the peripheral part of the portal acinus (central veins of the hepatic lobules).
Hepatocytes (Fig. 138. 8)
The hepatocytes are irregular shaped their quantity is about 60% of all cells present in the liver. The hepatocytes are 20-25 mm in length. They contain round nuclei. 20% of hepatocytes contain 2 nuclei. Durng a pregnancy the quantity of the cells, containing 2 nuclei, increases. The hepatocyte nuclei are 7-16 mm in diameter. The largest nuclei are polyploid. In old persons the polyploid nucleus number increases (80%). The hepatocyte cytoplasm is stained by acid or basic (alkaline) dyes. Every hepatocyte has the bile surface, directing to the biliary canaliculus, and vascular aspect, applying to the intralobular sinusoid. On both biliary and vascular surfaces of the hepatocyte there are microvilli.
The Golgi complex moves to the biliary surface when the bile is released into biliary canaliculi while it moves to the vascular surface when proteins, glucose and other substances are released into the blood vessels.
The hepatocytes contain both granular and smooth ER. On the granular ER the proteins synthesize, which move to the Golgi complex. On the smooth ER the glycogen and lipids are synthesized. In addition on the smooth ER toxins and drugs undergo detoxication. Therefore when toxins enter the organism, the smooth ER of hepatocytes undergoes hypertrophy.
Oval and thread-like mitochondria, containing few cristae diffusely scattered in the cell cytoplasm of hepatocytes. Lysosome and peroxisomes lie near the nucleus of the cell. Quantity of the incorporations (glycogen, lipids, pigments, and metabolism products) in hepatocytes undergoes alteration depending on the organism state (particularly depending on the nutrition).
In 3-5 hours after the nutrition in hepatocytes the glycogen begins storing (accumulation), in 10-12 hours its contents becomes maximum, in 24-48 hours the glycogen in the hepatocytes is absent. If the food contains much fat, in the lobule peripheral hepatocytes the lipid incorporations are seen. If the person suffers from some diseases (nuclear radial disease, cranial trauma, alcoholism, and others diseases), his liver undergoes obesity (includes much fat).
The liver function is rhythmic. At night the glycogen is synthesized in the central zone of hepatocytes. Later one is synthesized in the peripheral zone. At the daytime the bile is synthesized in the peripheral zone of the lobules, then in its intermediate zone. At last it is synthesized in the hepatocytes of the central zone of the lobules.
Bile Ducts
The bile ducts are divided into intra-hepatic and extra-hepatic ducts. The intra-hepatic ducts are biliary circum-lobular and interlobular ducts. The extra-hepatic ducts are left and right biliary ducts, which join to form common biliary hepatic duct. The cystic duct enters the latter to form the common bile duct. The interlobular biliary duct wall is lined with the cuboidal epithelium, lying on the basement membrane, surrounded by loose connective tissue.
The walls of the
left and right hepatic biliary ducts, cystic duct, and common bile duct (these
ducts are 3.5-
The mucosal layer consists of 2 laminae: 1) simple columnar epithelium and 2) proper mucosal lamina. The epithelial cells include lysosomes containing pigments. Among columnar epithelial cells some goblet cells are seen. The proper mucosal lamina is made up of loose connective tissue, which contains a number of elastic fibers and some mucosal glands.
The muscular tunic is thin. It consists of circular smooth muscle cell bundles. In the cyst duct wall and the common bile duct wall there are sphincters regulating the bile outflow into the duodenum. The adventitial layer consists of loose connective tissue.
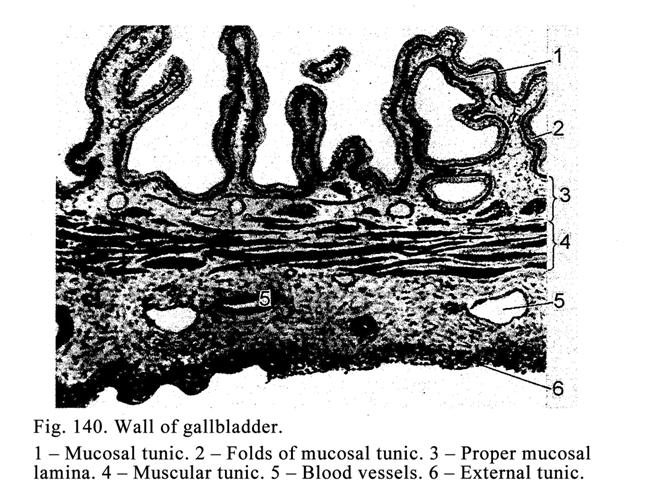
The Gallbladder
The gallbladder
volume is 40-70 ml its wall is 1.5-
The mucosal layer includes 2 laminae: 1) simple columnar epithelium with the striated border and 2) proper mucosal lamina, consisting of loose connective tissue, including number of elastic fibers. In the proper mucosal lamina there are tubular-alveolar glands (particularly in gallbladder cervix). Due to the striated border the epithelium absorbs the water from the cystic bile therefore this bile is darker than the hepatic bile.
The muscular tunic is made up of crossing smooth muscle cell bundles. The muscle cells of the tunic provide the cystic duct sphincter near the cystic cervix. The adventitial tunic consists of loose connective tissue rich in collagen fibers.
The Liver Nerve Supply
In the liver capsule there is the neural plexus consisting of sympathetic and parasympathetic nerve fibers ending by terminals on the blood vessel wall, biliary ducts, and hepatocytes.
Age Dependent Changes of the Liver
In an old person the lipofuscin quantity of the hepatocytes increases, the amount of the divisible hepatocytes decreases, quantity of the polyploid cells increases. The cell nuclei become hyperchromatic. The interlobular connective tissue layer becomes thicker.
The Liver Regeneration
The liver regenerates rapidly. After the liver removal in 50-70% the liver initial mass almost fully recovers in 2 weeks. The regeneration provides due to 1) hypertrophy of hepatocytes and 2) multiplication of hepatocytes. The food, containing much carbohydrates and proteins, stimulates the liver regeneration.
The liver provides 1) detoxication of toxins, drugs, alcohol, and hormones, 2) protective function (stellate macrophages engulf bacteria and others harmful substances), 3) participation in the carbohydrate metabolism, 4) synthesis of the blood proteins (albumin, fibrinogen, and others proteins), 5) the bile synthesis, 6) participation in the cholesterol synthesis, 7) accumulation of A, D, E, and K vitamins, 8) the blood accumulation, and 9) the blood formation (in embryonic life).
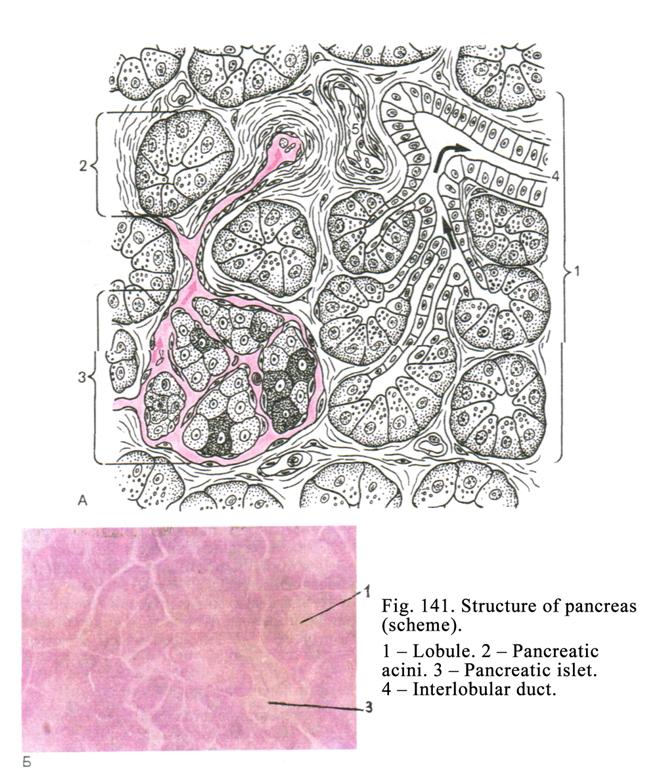
The Pancreas
The pancreas (Fig. 141) includes 2 parts: 1) exocrine part and 2) endocrine part.
The pancreas exocrine part produces secretion, which contains enzymes (trypsin, chymotrypsin, amylase, lipases etc.). The secretion is discharged into the duodenum.
The pancreas endocrine part produces hormones (insulin, glucagon, somatostatin, VIP, and pancreatic polypeptid).
The Pancreas Development
The pancreas is derived from 2 sources: 1) the epithelial cells are derived from the intestine endoderm bulges (one dorsal and two ventral) entering the mesentery and 2) the connective tissue, blood vessels, and capsule are derived from mesenchyme. At the third month of the embryonic life the exocrine and endocrine parts of pancreas undergo differentiation. At the same time exocrine part terminal regions and ducts are formed. On the surface of the ducts the bulges appear, which separate from the ducts to form pancreatic islets.
Preliminary Remarks
The thin capsule, fusing with peritoneum, covers the pancreas. Septa, extending into the gland parenchyma, divide it into lobules. In connective tissue septa there are interlobular ducts, blood vessels, parasympathetc nerve ganglions, and lamillar corpuscles. The exocrine mass part is 97% the endocrine mass part is only 3%.
The Exocrine Region of the Pancreas
Acini, intralobular ducts, falling into the interlobular ducts, running into the excretory duct, represent the exocrine region of the pancreas. The structural and functional unit of the exocrine region of the pancreas is the pancreatic acinus. The acinus includes the terminal region and intercalated duct invaginating into the terminal region. The acinus is sack shaped its length is about 100-150 mm. Between acini there are thin layers of connective tissue containing reticular fibers, capillary blood vessels, nerve fibers, and autonomic nervous ganglions. The acinus cells called exocrine cells of the pancreas (or acinocytes) lie on the basement membrane. In the pancreatic acinus center the intercalated duct cells are seen. These are called centroacinar epithelial cells.
The acinocytes are pyramid shaped. Their wide basal end lies on the basement membrane while the thin apical end reaches the acinus lumen. The basal end cell membrane form folds the cell apical end bears microvilli. The tight junctions, desmosomes, and digiform intercellular junctions join acinocytes with each other. In the apical part of the cells there are zymogene granules, which are about 800 nm in diameter. The cell apical part, stained by acid dyes is called the zymogene zone. The acinocyte basal part, containing a number of rough ER, ribosomes, stained by the basic (alkaline) dyes, is called the homogenous zone. The cell mitochondria are scattered in the cytoplasm the Golgi complex lies over the nucleus. The round nucleus of cell lies in its basal part. The nucleus contains nucleoli.
The acinocytes
release enzymes (pepsin, lipase, amylase and other).
Pancreas Ducts
The intercalated duct can invaginate into the acinus center (in this case in the center of the acinus centroacinar cells are seen). The intercalated duct can also apply to lateral surface of the acinus (in this case the duct cells lie on the same membrane that the acinocytes). The centroacinar cells are small. Surround their oval nucleus thin layer of slightly stained cytoplasm are seen. Organelles of the cells are scanty. The cell apical surface bears some microvilli.
The intercalated duct falls into duct lying between acini (inter-acini duct). The cuboidal epithelial cells, containing prominent Golgi complex, line the inter-acini duct. The desmosomes join the cells with each other. The cell free surface bears microvilli. These cells probably release the fluid component into the pancreas secretion. The inter-acini ducts fall into intralobular ducts, lined with cuboidal epithelial cells, containing round nuclei, and poorly developed organelles (Golgi complex, mitochondria, ribosomes, and smooth ER). The ducts run into the interlobular ducts. The interlobular ducts, running in the connective tissue septa, fall into the excretory duct. The columnar epithelium lines the interlobular and excretory ducts. Among the columnar cells there are goblet cells and endocrine cells releasing the pancreozymin and cholecystokinin. Deep to the epithelium the proper mucosal lamina of the duct lies.
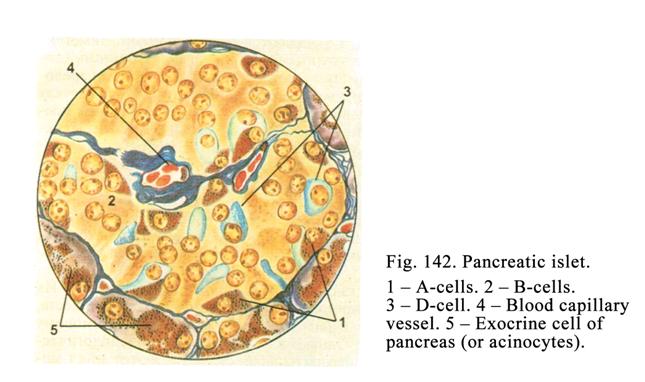
Endocrine Region of the Pancreas
The endocrine part of the pancreas is in the form of numerous rounded collections of cells called the pancreatic islets (Fig. 142). Their quantity is about 1-2 millions. They are most numerous in the tail of the pancreas. The islets have various shapes (predominantly oval or round). They are 100-300 mm in diameter. The islets consist of endocrine cells. The cells are smaller than those of the acinus. They have slightly stained cytoplasm, Golgi complex, rough ER, mitochondria, and secretory granules. Depending on the structure and the contents of the granules there are 5 types of endocrine cells in islets: 1) B-cells, 2) A-cells, 3) D-cells, 4) D1-cells, and 5) PP-cells.
B-cells lie in the islet center their quantity is about 70%. B-cell granules are 275 nm in diameter they are soluble in the spirit but not in water. In the center of the granules the dense body is seen. The light ring surrounds the body. The granules, stained by basic dyes (aldehyde fuchsin), contain the hormone insulin (sometimes contain Zn). The B-cells function is the release of the insulin.
A-cells predominantly lie in the islet peripheral part they form about 20%. The granules of cells are about 230 nm in diameter. They are soluble in water but not in spirit. Acid dyes (acid fuchsin) stain the granules. The granule central part contains the dense body surrounded by a light ring. A-cell granules contain the hormone glucagon, which splits glycogen, which is in the hepatocytes and muscle tissue, to form glucose entering the blood, to provide the hyperglycemia.
D-cells are pear shaped or star shaped. They lie in the peripheral part of the islets. These cells constitute about 5-10% from the islet cells. Their granules are about 325 nm in diameter. In the granules the light ring is absent. The granules contain the hormone somatostatin, which inhibits the release of insulin from B-cells and glucagon from the A-cells, and enzymes from acinocytes.
D1-cells constitute about 2-5% from the islet cells. Their argentophil granules are about 160 nm in diameter. In the granules there is the light ring. The granules contain vasoactive intestinal polypeptid (VIP), which lowers the blood pressure and stimulates the release of enzymes and hormones from the pancreas.
PP-cells (2-5%) lie in the islet peripheral part their granules are 140 nm in diameter. PP-cells release the pancreatic polypeptid stimulating discharge of the secretions of the stomach and pancreas.
Intermediate Cells
They contain granules similar to those of acinocytes and granules similar to those of the islet cells (A-cells, B-cells, and D-cells). If the cells contain A type granules, they are called A intermediate cells etc. The intermediate cells locate between the acini and islets. These cells release their secretions into blood vessels and ducts. For example, the cell trypsin granules, poured out from the intermediate cell into the blood vessel, reach the islet B-cells and stimulate the release of the insulin from them.
The Pancreas Blood Supply
The upper celiac artery branches enter the interlobular septa of the pancreas (interlobular arteries). There are 2 versions of the pancreas blood supply. According to the first version the interlobular arteries run along the ducts, reach the acini, and undergo the division into the arterioles and capillary vessels, enter the islets, and surround the acini. Then the capillary vessel blood pours in the venules, then the veins running along the arteries.
According to the second version the arterioles (called the afferent arterioles) branch to form the capillary network entering the islets. Then capillary vessels run into efferent arterioles running to the acini, and branch to form the capillary network surrounding the acini. From the capillary network the blue blood pours into the venules running into the veins. The veins enter the portal vein of the liver.
Lymphatic Vessels of the Pancreas
The blind lymphatic capillary vessels, surrounding acini and islets, run into larger lymphatic vessels running along the blood vessels.
The Pancreas Nerve Supply
Efferent sympathetic and parasympathetic nerve fibers regulate pancreas function. Afferent nerve fibers are also present. In connective tissue septa there are parasympathetic neural ganglions. The efferent sympathetic nerve fibers are axons of the sympathetic efferent neurons of the sympathetic ganglions. Parasympathetic neural fibers are axons of efferent cells (Dogels I type cells) located in the parasympathetic neural ganglion. The vagal nervous fibers travel to the parasympathetic effector neurons (Dogels I type cells) to form the synapse. The axons of Dogels I type cells end with motor terminals (on smooth muscle cells) or neurosecretory terminals (on glands).
The afferent nervous fibers are dendrites of the nervous ganglion sensitive neurons ending with receptors (sometimes end with lamellar corpuscles).
Age Dependent Changes of the Pancreas
In on old person the quantity of the islets decreases, the renewal of cell organelles becomes low; the pancreas exocrine region function becomes lower.
The Pancreas Regeneration
The dead islet cells are not replaced therefore the quantity of the islet cells may decrease.
CHAPER
23
THE
RESPIRATORY APPATATUS
The respiratory system consists of the lungs and the passages through which air reaches them. The respiratory passages include the nasal cavities, the pharynx, the laryngeal cavity, the trachea, and the bronchi lined by some types of epithelial cells (Fig. 143).
The Respiratory Apparatus Development
The connective tissue, smooth muscle, cartilage tissue, and bones are derived from the mesenchyme. The pleural mesothelium is derived from the mesoderm. The epithelium of the laryngeal cavity, trachea, bronchi, and lungs is derived from the anterior endoderm gut projection. The projection arises from the gut at the 4th week of the embryo life. This projection is divided into the right and left halves, from which epithelial lining of bronchi is formed. From surrounding mesenchyme, the connective tissue, smooth muscle, and cartilage of bronchi are formed. Before the 7th month of the embryonic life the bronchioles and pulmonary alveoli are formed. The alveolar epithelial cells are cuboidal. The alveoli are collapsed. After the birth the newborn begins breathing and the alveoli are extended and the alveolar epithelial cells become flat.
The Nasal Cavities
The cavities include the nasal vestibule and proper nasal cavities. The nasal vestibule is lined with the skin, which is the continuation of that on the exterior of the nose. The proximal part of the nasal vestibule is lined with the stratified squamous non-keratinized epithelium. In the proper mucosal layer there are hair roots and sebaceous glands. The nasal hairs trap the dust corpuscles so inhaled air is pure.
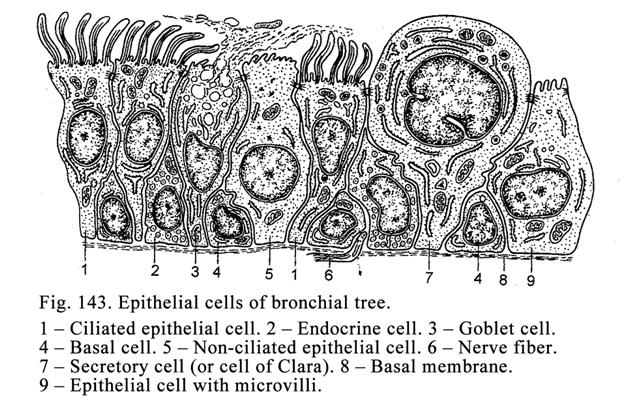
The mucosal layer consisting of 2 laminae lines the proper nasal cavities: 1) pseudostratified epithelium and 2) proper mucosal lamina. The pseudostratified epithelium includes ciliated cells, non-differentiated cells, cells with microvilli, and goblet cells.
The proper mucosal lamina is represented by loose connective tissue containing numerous elastic fibers. In the proper mucosal lamina there are mucosal gland terminal regions and lymph nodules forming the tubular tonsils. Deep to the basal lamina epithelium there is the dense capillary network. The capillary network blood regulates the inhaled air temperature (if the air is cold, the blood warms it, if the air is hot, the blood colds it). In the proper mucosal lamina there are the arterial and venous plexuses. The vein wall contains many muscle cells. The venous plexus vein wall is thin in the lower concha. If these veins are filled with blood, the mucosal layer becomes swollen so the respiration is hampered. The nose lymphatic vessels join with those of the large salivary glands, perivascular spaces of brain, and subarachnoid space.
On the superior concha and partly on the middle concha the olfactory mucosa locates.
The paranasal air sinuses (maxillary and frontal sinuses) are lined with the mucosal layer, which is similar to that of the nasal cavities but the air sinus mucosal layer is thinner, than the nasal mucosal layer.
The nasal cavity nerve supply is provided by the nerve fibers of the trigeminal nerve. The afferent nerve fibers end with mechanoreceptors, thermoreceptors, and chemoreceptors.
The Pharynx
Through the pharynx the respiratory and alimentary passages run. The pharynx wall consists of 4 layers: 1) mucosal layer, 2) submucosal layer, 3) muscular tunic, and 4) adventitial layer. The pharynx includes the oropharynx, laryngo-pharynx, and nasopharynx parts.
The mucosal layer of the oropharynx and laryngo-pharynx is lined with the stratified squamous non-keratinized epithelium but the pseudostratified epithelium lies in the nasopharynx. In the proper mucosal lamina, consisting of the loose connective tissue, there are prominent elastic fibers.
The submucosal layer consists of the loose connective tissue in which mucous compound glands are lodged. The muscular tunic consists of the internal longitudinal layer and the external circular layer of the striated musculature. The adventitial layer is made up of loose connective tissue.
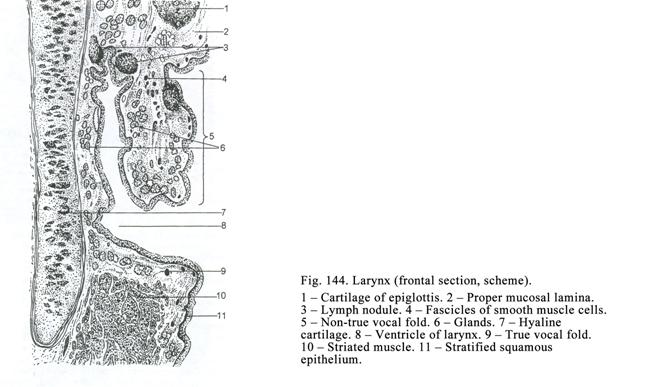
The Larynx
The larynx wall includes 3 layers: 1) mucosal layer, 2) fibro-cartilaginous tunic, and 3) adventitial layer (Fig. 144).
The mucosal layer is made up of 2 laminae: 1) epithelial lamina and 2) proper mucosal lamina. The stratified squamous non-keratinized epithelium forms the epithelial lamina of the mucosal layer vocal folds. The pseudostratified epithelium forms the rest part of the mucosal layer epithelial lamina.
The proper mucosal layer includes numerous elastic fibers. In this layer there are the lymph nodule aggregations, forming laryngeal tonsil, and seromucous gland terminal regions.
The mucosal layer covers the true vocal folds and pseudo-vocal folds. Within the true vocal folds there are a number of the elastic fibers and striated muscle fibers. When the muscle fibers contract, the space between the true vocal folds becomes narrow when the muscle fibers relax, the space becomes wide. The pseudo-vocal folds contain the elastic fibers and smooth muscle cells only.
Deep to the basal membrane there is the dense capillary network regulating the temperature of the inhaled air. The connective tissue subjacent to the epithelial lining of the vocal folds is devoid of lymphatic vessels. This factor shows that the cancer doesnt give any metastases.
The fibro-cartilaginous tunic is the larynx skeleton consisting of the hyaline and elastic cartilages. The adventitial layer consists of the loose connective tissue.
The Epiglottis
The epiglottis separates the larynx from the pharynx. It is made up of the elastic cartilage covered by the mucosal layer. Its pharyngeal and laryngeal surfaces are lined with the stratified squamous non-keratinized epithelium. The epiglottis closes the larynx at the time of swallowing.
The larynx provides 1) air passage, 2) voice production, and 3) inhaled air temperature regulating.
The
Trachea
It is tube running from the cricoid cartilage to the trachea bifurcation. The trachea wall (Fig. 145. 2) includes 4 layers: 1) mucosal layer, 2) submucosal layer, 3) fibro-muscular cartilaginous tunic, and 4) adventitial tunic.
The Mucosal Layer
It consists of 2 laminae: 1) pseudostratified columnar epithelium and 2) proper mucosal lamina.
The pseudostratified columnar epithelium includes 5 cell types: 1) ciliated epithelial cells, 2) goblet cells, 3) basal (non-differentiated) cells, 4) endocrine cells, and 5) antigen presenting cells (macrophages).
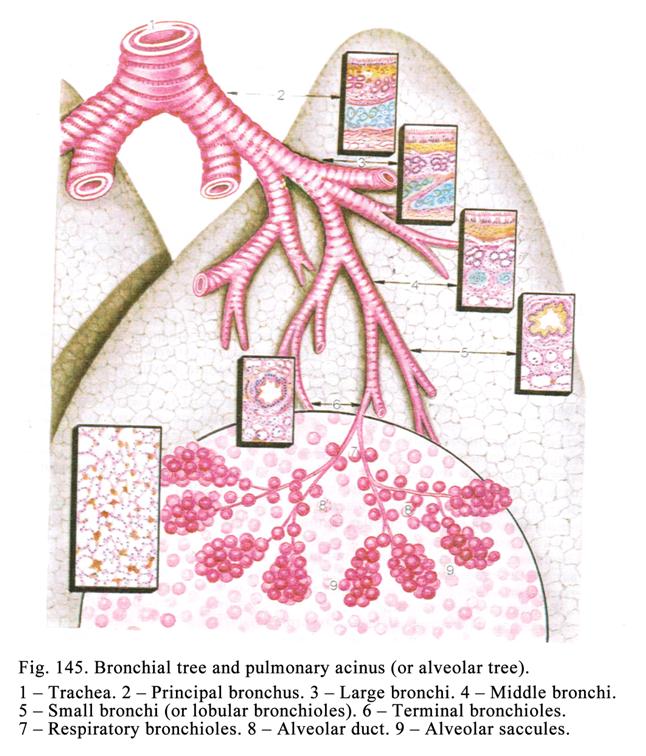
Ciliated epithelial cells are the tallest cells of the epithelium lining of the trachea lumen. The cell narrow basal part is on the basement membrane the cell apical wide surface bears cilia (Fig.143 1). The cilia are 5 mm in length. They (cilia) provide the oscillatory movement so the dust particles, trapped by mucous, are removed by the cilia towards the larynx and pharynx.
The temperature 18-330 is optimum for the oscillation of the cilia but the lower or higher temperature inhibits the oscillation. The inhaled smoke temperature is about 500 therefore the oscillation of the cilia stops so the dust particles and bacteria accumulate on the trachea mucosal lamina. It leads to the acute tracheitis and bronchitis and at last to chronic tracheitis and bronchitis. These diseases are precursors of the respiratory system cancer. The American scientists show that the smoking persons more often suffer from the lung cancer (in 15 times) than persons who do not smoke.
The goblet cells (Fig. 143 3) structure is similar to that of the gut goblet cells but the former synthesize the secretion containing hyaluronic and sialic acids. The acid is known to kill bacteria.
The mucous secretion, lining the mucous membrane of trachea, contains the immunoglobulin A (IgA). The protein component of the immunoglobulin is produced in the plasma cells while carbohydrate component in the epithelial cells of the mucous membrane. Due to the immunoglobulin the immune reaction is provided on the mucosal layer.
The basal epithelial cells (Fig 143 4) are cone shaped cells. They are short their basal part is wide and lies on the basement membrane. These are undifferentiated cells. They replace the cells, which die.
Endocrine cells contain Golgi complex, rough ER, mitochondria, and secretory granules. These cells produce calcitonin, serotonin, dopamine, noradrenalin, and others hormones providing the muscle contraction of the respiratory passages.
Antigen presenting cells (cells of Langherhans) are dendritic cells. They contain lobular or oval nucleus, organelles (particularly lysosomes), and special granules, which are racket shaped. On the cell surface there are receptors to the fragments of immunoglobulin G (IgG) and receptors to the fragments of the C3 complements.
The antigen presenting cells perceive the allergic antigens; release the factor, providing the cancer cell necrosis, discharge cytokines, stimulating the multiplication and differentiation of lymphocytes. These cells along with the lymphocytes constitute the immune system of the respiratory system passages.
The proper mucosal lamina consists of loose connective tissue containing a number of longitudinal elastic fibers. In the lamina there are lymph nodules, tracheal gland ducts, single smooth myocytes, and dense capillary network regulating inhaled air temperature.
The Submucosal Layer
It consists of the loose connective tissue. Here there are the tracheal seromucous gland terminal regions.
The Fibro-muscular Cartilaginous Tunic
This consists of 16-20 cartilages. Each of these is C-shaped mass of hyaline cartilage. The open end of the C is directed posteriorly. The gaps between the cartilage ends, present on the posterior aspect, are filled in with fibrous tissue and the smooth muscle to form the tracheal muscle. The connective tissue along with the muscle forms the soft posterior tracheal wall attaching to the esophagus. It facilitates the food movement through the esophagus.
The Adventitial Tunic
It is made up of loose connective tissue. Its collagen fibers extend into the mediastinum.
The Trachea Blood Supply
In the trachea wall mucosal layer there are arterial and venous plexuses. In the subepithelial connective tissue there is the blood capillary vessel plexus regulating the inhaled air temperature. In the mucosal layer connective tissue the lymph capillary plexus is present.
The Trachea Nerve Supply
Two nervous plexuses provide innervation of the trachea wall: 1) the plexus consisting of sympathetic and parasympathetic nerve fibers and 2) plexus including afferent nerve fibers (the sensitive ganglion neuron dendrites).
The trachea provides air passage and regulation of the inhaled air temperature.
The Lungs
These include the bronchial tree and respiratory part (alveolar tree).
The Bronchial Tree
The bronchial tree ((Fig. 145) belongs to the respiratory passages of the lung, including principal bronchi, arising from the bifurcate (large caliber), 2 lobular bronchi (large caliber), 4 zonal bronchi (large caliber), 10 segmental bronchi (middle caliber), sub-segmental bronchi (fourth and fifth sub-segmental bronchi of middle caliber), small bronchi (or lobular bronchioles), and terminal bronchioles.
The Structure of Bronchi of Large and
Middle Calibers
The wall of these bronchi (Fig. 145. 3 & 4) includes 4 layers: 1) the mucosal layer, 2) the submucosal layer, 3) the fibro- cartilaginous tunic, and 4) the adventitial tunic.
The Mucosal Layer
It includes 3 laminae: 1) epithelial lamina, 2) proper mucosal lamina, and 3) muscular lamina of mucosa.
The epithelial lamina is made up of the pseudostratified epithelium including ciliated, endocrine, basal, antigen presenting, and goblet cells. As the bronchi get smaller the epithelium lamina becomes thinner and quantity of the goblet cells decreases.
The proper mucosal lamina consists of the loose connective tissue containing a number of the longitudinally directed elastic fibers. The lamina includes single lymph nodules belonging to the immune system of the respiratory passages. The subjacent connective tissue contains the dense blood capillary vessel network.
The muscular lamina of mucosa consists of the circularly directed smooth muscle cells. As the bronchus diameter becomes smaller the muscular lamina becomes relatively thicker.
The Submucosal Lamina
It is made up of loose connective tissue containing the seromucous gland terminal regions.
The Muscular Fibro-cartilaginous Tunic
The tunic consists of the fibrous tissue and cartilages. The principal bronchus cartilage is a C-shaped hyaline cartilage. In the wall of the lobar and zonal bronchi there are the hyaline cartilage plates. The wall of the middle bronchi contains plates (islets) of the elastic cartilage.
The Adventitial Tunic
The tunic is made up of loose connective tissue. Its collagen fibers extend into the interstitial connective tissue of the lungs.
The Small Bronchi (Lobular Bronchioles)
The small bronchial wall (Fig. 145. 5) consists of 2 layers: 1) mucosal layer and 2) adventitial tunic.
The mucosal layer is made up of 3 laminae: 1) epithelial lamina, 2) proper mucosal lamina, and 3) muscular mucosal lamina.
The epithelial lamina consists of two-stratified or simple columnar epithelium. Amongst the epithelial cells the goblet exocrine cells are absent.
The proper mucosal lamina is made up of loose connective tissue containing a number of elastic fibers.
The muscular mucosal lamina consists of the relatively thick layer of the circular smooth muscle cells. Due to the muscular mucosal lamina and the absence of the cartilage in the bronchial wall the mucosal layer forms deep folds shrinking the bronchial lumen. When the muscular mucosal lamina is contracted the respiration becomes difficult.
The Terminal Bronchiole
The terminal bronchiole wall includes 2 thin layers: 1) mucosal layer and 2) adventitial tunic.
The mucosal layer is made up of 3 laminae: 1) epithelial lamina, 2) proper lamina of mucosa, and 3) muscular lamina of mucosa.
The epithelial lamina consists of the simple cuboidal ciliated epithelium. Amongst the epithelial cells there are the secretory cells of Clara, brush cells, and non-ciliated cells (Fig. 143).
The secretory cell of Clara (Fig. 143. 7) has the narrow basal part and wide apical end. The cell basal part lies on the basement membrane. The cell contains the round nucleus, Golgi complex, smooth ER, mitochondria, and secretory granules. The cell of Clara releases the lipoproteins and glycoproteins (components of pulmonary surfactant) it also releases enzymes destroying the inhaled harmful substances and toxins.
The brush cells (Fig. 143. 9) are O-shaped i.e., their apical and basal parts are narrow while their middle part is wide. The cells contain round nucleus and usual organelles their apical part bears the brush. The brush cells are sensitive to the smell.
The non-ciliated cells (Fig. 143. 5) are column shaped. They are somewhat taller than other cells. Their cytoplasm contains Golgi complex, mitochondria, ER, secretory granules, and the glycogen inclusions. Their function is not known.
The
Lung Respiratory Part
They provide the exchange of the oxygen and carbon dioxide between the lung alveolar air and the blood.
The Alveolar Tree (The Pulmonary Acinus)
The pulmonary acinus (Fig. 146) is a structural and functional unit of the lungs. The primary respiratory bronchiole is the initial part of the lung acinus. It (respiratory bronchiole) is divided into 2 the secondary respiratory bronchioles. The latter are divided into 2 the tertiary respiratory bronchioles each of them is divided into 2 alveolar ducts each of them is divided into 2 alveolar saccules. In the wall of the respiratory bronchioles, alveolar ducts, and alveolar saccules there are pulmonary alveoli.
Thus, the ramification of the primary respiratory bronchioles, alveolar ducts, and alveolar saccules, including alveoli in their walls, is the pulmonary acinus.
The pulmonary acini are separated from each other by the connective tissue. 12-18 pulmonary acini constitute the pulmonary lobule separated from each other by loose connective tissue.
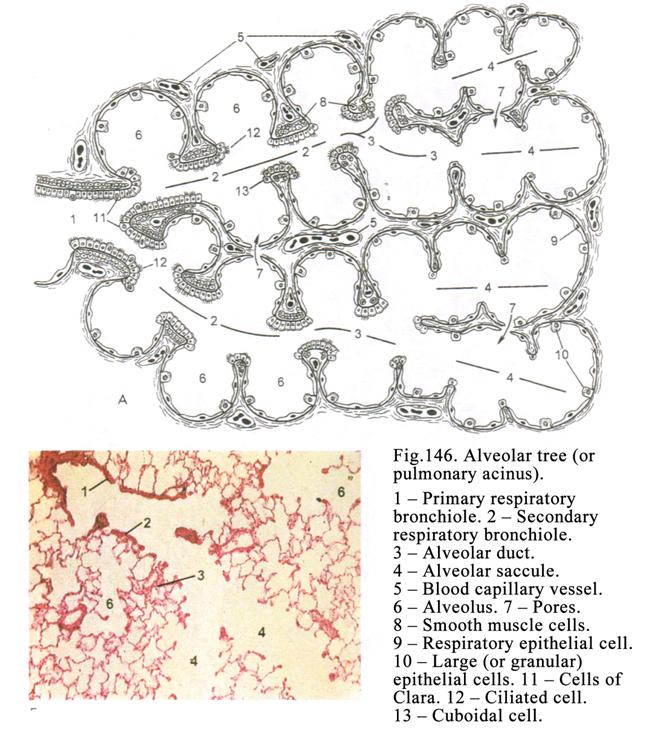
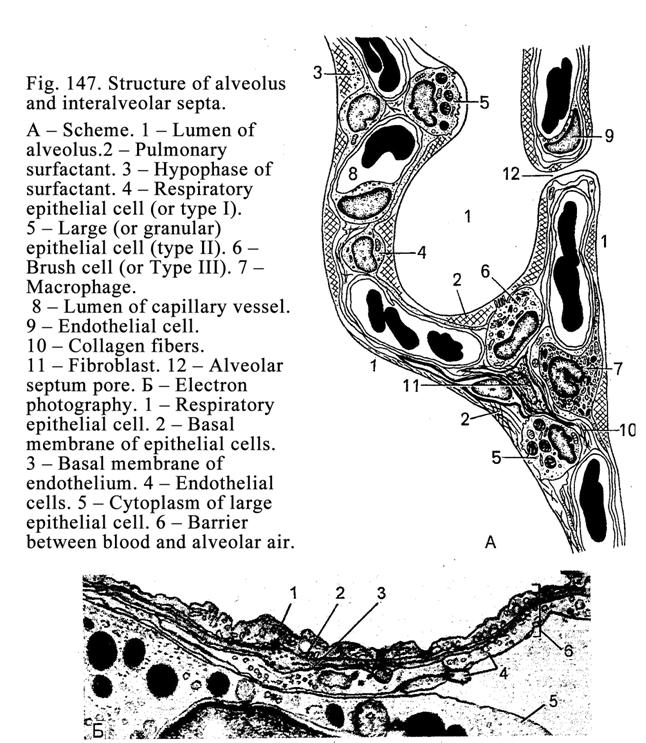
The Respiratory Bronchiole Wall
It is thin and includes 2 weakly formed layers: 1) mucosal layer and 2) adventitial tunic.
The mucosal layer of the respiratory bronchioles is lined with the simple cuboidal non-ciliated epithelium in which some ciliated cells and Clara cells are sometimes seen. The proper lamina of the mucosa is thin the muscular mucous lamina includes single circular smooth muscle cells.
The respiratory bronchiole adventitial tunic consists of a thin layer of connective tissue. Its collagen fibers extend into the inter-alveolar connective tissue. The respiratory bronchiole wall includes single alveoli while the wall of alveolar ducts and alveolar saccules is made up of alveoli.
The Alveoli
The alveoli (Fig. 147) are vesicles (120-140 mm in diameter) opened into the lumen of the respiratory bronchioles, alveolar ducts, and alveolar saccules. Between the alveoli there are the thin connective tissue septa (2-8 mm in thickness), containing the elastic and thin collagen fibers, forming network, fibroblasts, mast cells, and antigen presenting cells (macrophages). In the inter-alveolar septa there are capillaries (5-7 mm in diameter), which occupy 75% of the every alveolus surface. The alveoli communicate with each other by the alveolus septum pores, which are 10-15 mm in diameter.
The alveolar wall, lined with the simple squamous epithelium, consists of the basement membrane supported by thin collagen and reticular fibers. The simple squamous epithelium cells are divided into 2 basic types: 1) respiratory epithelial cells and 2) large secretory epithelial cells. In the alveolar wall and its surface there are some macrophages.
Respiratory epithelial flat cells contain a few mitochondria and pinocytotic vesicles their apical surface bears microvilli. The cell thickness, where the nucleus is present, is about 5-6 mm while the thickness of the rest cell part is about 0.2 mm. Opposite the respiratory epithelial cell the rest cytoplasm (without any nucleus) the capillary endothelial cell cytoplasm (free from the nucleus) localizes. In the same place the thickness of barrier between the alveolar air and blood of capillary vessel is about 0.5 mm. The barrier includes the cytoplasm respiratory epithelial cell without any nucleus, the alveolus basement membrane, thin connective tissue, the blood capillary vessel membrane, and endothelial cell cytoplasm without any nucleus. The respiratory epithelial cells provide the exchange of oxygen and carbon dioxide between the alveolar air and hemoglobin of erythrocytes.
The large epithelial secretory (cuboidal or oval) cells constitute about 5% (the respiratory epithelial cells constitute about 95%). The cell free surface bears microvilli. The cell cytoplasm contains Golgi complex, ER, ribosomes, mitochondria, and secretory granules that appear to be made up of several layers (they are therefore called multilamellar bodies). These bodies are the markers of the large epithelial cells. The large epithelial cells are believed to produce components (phospholipids and proteins) of the surfactant.
The Surfactant of the Alveoli
The surfactant (Fig. 148), lining the luminal surface of the alveolar epithelial cells, includes 3 components: 1) membrane-like component, containing phospholipids and proteins, synthesized by large epithelial cells, 2) the fluid component, containing lipoproteins and glycoproteins, released by the cells of Clara, and 3) reserve component. The surfactant prevents 1) the adhesion (collapse) of the luminal surface of the alveoli after the expirations, 2) the passage of bacteria from the alveoli to the interstitial tissue of the lung, and 3) the fluid (transudate) transport from the interstitial tissue to the alveoli.
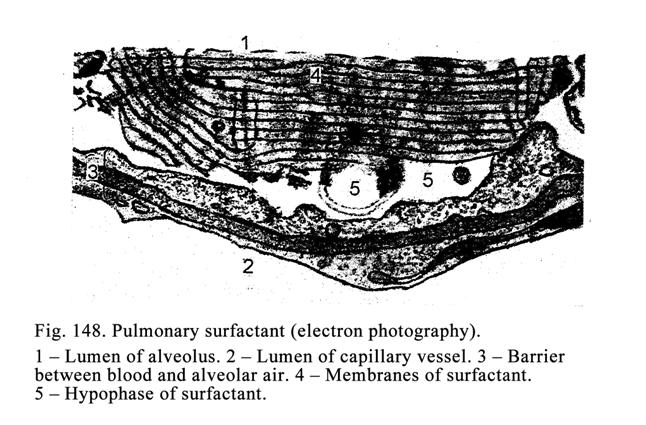
The alveolar macrophages are dendrite cells, containing an oval nucleus and well-developed lysosomes, situated in the alveolus wall or on its surface. The macrophages can migrate from the alveoli into the interstitial connective tissue. In their cell cytoplasm there are incorporation of the lipids, which oxidize to produce the heat worming the inhaled air (the inhaled air temperature must be similar to that of the human body). The alveolar macrophages provide the protective function (they engulf bacteria, dust particles, and surfactant). These cells take part in the fat exchange and produce the heat energy.
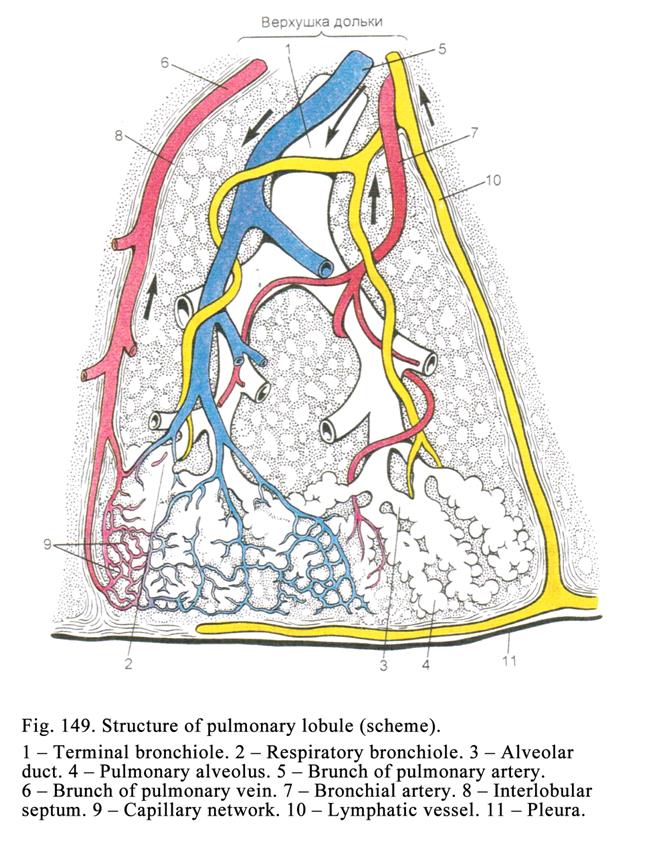
The Lung Blood Supply
The lung artery and bronchial artery enter the lung. In lung artery the blue blood flows. This artery ramifies run along the bronchi (Fig. 149). When it reaches the alveoli, the branches form the capillary network surrounding the alveoli. The network capillaries are 5-7 mm in diameter. Every capillary vessel applies to two adjacent alveoli. The erythrocytes align in one row within the capillary lumen. Both these factors facilitate the exchange of gases between the alveolar air and the hemoglobin of the erythrocytes. The capillary vessel blood, leaving the carbon dioxide and receiving the oxygen, goes into the pulmonary vein entering the left atrium.
Bronchial arteries are the aorta branches. They brunch and run along the bronchi and provide their walls and lung tissues with oxygen and nutrients. In the wall of the bronchi the artery branches form the arterial plexuses in the mucosal and submucosal layers in the bronchus wall. The plexus arterioles branch to form the dense blood capillary network deep to the basement membrane. The capillary vessels run into venules, carrying blue blood into small veins, falling into the anterior and posterior bronchial veins. In the level of the small bronchi there are arteriovenous anastomoses between the arterioles of the bronchial arteries and venules of the lung veins, through which the oxygenated blood returns into the heart.
The Lung Lymphatic Vessels
In the lung there are the superficial and deep lymphatic plexuses. The superficial plexus localizes in the pleura while the deep plexus (Fig. 149) is situated in the loose connective tissue surrounding pulmonary acini, lobules, and near bronchi and blood vessels. The bronchial wall has one lymphatic plexus in its submucosal layer and another one in proper mucosal lamina.
The Lung Nerve Supply
The lung nerve plexuses locate in the connective tissue surrounding blood vessels and bronchi. The plexuses include parasympathetic nerve ganglions, efferent (sympathetic and parasympathetic) nerve fibers and afferent nerve fibers. The sympathetic nerve fibers are sympathetic ganglion efferent neuron axons ending with the neuromuscular terminals on the smooth muscle or with neurosecretory terminals on glands.
The parasympathetic nerve fibers are the parasympathetic ganglion neuron (Dogels cell type I) axons ending with neuromuscular or neurosecretory terminals. These efferent neurons (Dogels type I cells) receive impulses from the vagal nerve fibers. The sympathetic nerve fiber stimulation makes the blood vessels shrink and the bronchi dilate while the parasympathetic nerve fiber stimulation makes the blood vessels dilate and the bronchi shrink so the respiration becomes difficult.
The afferent nerve fibers are dendrites of the ganglion sensitive neurons. The afferent neuron fibers end with receptors on the bronchial wall or lung parenchyma.
Age Dependent Changes of the Respiratory
System
In children the quantity of the alveoli and elastic fibers increases and reaches its maximum in the puberty. In on old person quantity of the alveoli and elastic fibers are destroyed while the connective tissue, predominantly containing collagen fibers, grows. These changes lead to the lung dilation (emphysema) and the gas exchange decreases.
The Pleura
The pleura covering the lung, is called the visceral pleura while the pleura, covering the thorax wall, is called the parietal pleura. It is made up of the connective tissue wich covered with the mesothelium. The visceral pleura connective tissue contains more smooth muscle cells and elastic fibers than the parietal pleura. The visceral pleura connective tissue fibers extend into the lung connective tissue stroma. Depending on the lung movement the pleura mesothelium assumes the various shapes. During the inspiration the mesothelial cells become flat while during the expiration they become cuboidal. The lung provides respiratory function (the gases exchange) and non-respiratory functions.
Non-respiratory Functions
The respiratory system 1) regulates the inhaled air temperature, 2) moistens the inhaled air, 3) purifies inhaled air from the bacteria, dust corpuscles, and others harmful components, 4) provides the immune protection, 5) takes part in the exchange of lipids, salts, and water (every day through the lungs 500 ml of water is losed), 6) takes part in the regulation of the blood clotting system due to the lung mast cells, 7) provides the hormone secretion (calcitonin, noradrenalin, dopamine, serotonin, and others hormones), 8) inactivates the bradykinin and the serotonin due to the monoaminoxydase contained in the macrophages and mast cells, 9) synthesizes lysozyme, interferon, pyrogen (by the macrophages), 10) destroys the small clot and the carcinoma cells (within the lung blood vessels), 11) accumulates the blood (in the lung blood vessels), 12) takes part in the voice produce, 13) responses the smell reception, and 14) takes part in releasing some substances (ammonia, acetone, and alcohol).
CHAPTER
24
SKIN AND ITS APPENDAGES
The skin consists of the dermis and epidermis (stratified squamous keratinized epithelium) covering the skin surface. Below the dermis the subcutaneous layer lies.
The Skin Development Origins
The epidermis cells
(keratinocytes), skin appendages (nails, hairs, sebaceous glands, sweat glands,
and breasts) are derived from the ectoderm, melanocytes and cells of Merkel are
derived from the nervous crest; dendritic cells (macrophages) are derived from
monocytes. The skin connective tissue is derived from mesoderm.
The Epidermis
There are thick epidermis and thin epidermis. The thick epidermis (600 mm) covers the palms and ventral surface of the fingers and the corresponding surface of the feet. The thin epidermis is on the rest skin surface. The thinnest epidermis is on the face and head.
The Thick Epidermis Structure
This is about 50 cells thick forming 5 basic layers: 1) basal layer, 2) spinous layer, 3) granular layer, 4) lucid layer, and 5) cornified layer (Fig. 150). The lucid layer of the epidermis of the rest skin is absent.
The basal layer includes 4 species of cells: 1) keratinocytes, 2) melanocytes, 3) cells of Merkel, and 4) macrophages (dendritic cells of Langherhans).
The keratinocytes constitute about 85%. They rest on the basement membrane. These cells are column shaped, joined with each other by desmosomes, joined with the basement membrane by hemidesmosomes. The basal keratinocyte cytoplasm, stained by the basophil dyes, contains the oval nucleus in the cell basal part. In the cell cytoplasm there are usual organelles. On the rough ER the keratin protein molecules are synthesized, from which the filaments are formed. In the keratinocyte cytoplasm the pigment granules are seen, which have engulfed by the keratinocyte.
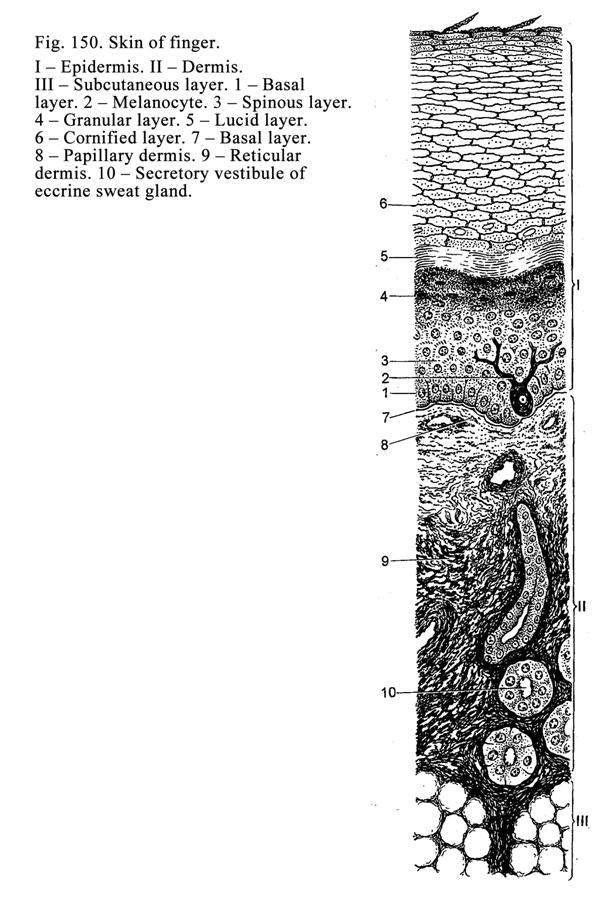
Amongst the basal layer keratinocytes there are stem cells present in the G0 period of the cell cycle. Periodically the stem cells enter the cell cycle and undergo the mitotic division. The formed daughter cells continue dividing and undergo the differentiation. Due to the keratinocytes division the entire renewing of the keratinocytes is provided in 3-4 weeks. Therefore the basal layer is called the germinal layer. The part of the daughter keratinocytes migrates into the spinous layer.
The keratinocytes provide germinal function, synthesis of keratin, thymosin, and thymopoietin stimulating antigen undependent differentiation of the T-lymphocytes.
The melanocytes are branched cells their cytoplasm is slightly basophilic. The cell cytoplasm contains synthetic apparatus (Golgi complex, ER, and mitochondria), pigment granules, and enzymes (dihydroxy-phenylalanine or DOP enzyme and other) taking part in the pigment synthesis. The pigment is discharged from the cells by the exocytosis. The melanocytes are large cells therefore their processes extend into the spinous layer. About 10% of cells seen in the basal layer are melanocytes.
The cells of Merkel (Fig.150) are shorter and wider than keratinocytes. The cells contain an irregular shaped nucleus, secretory granules, including VIP, encephalin, and other substances. Their cell cytoplasm is slightly stained. Sensory nerve endings are present in relation to these cells. Between the nerve ending and the cell there is the disc of Merkel.
The cells of Merkel provide 1) endocrine function (release the encephalin and others hormones), 2) participation in the regeneration of the epidermis, 3) participation in the tonus and permeability of the blood vessel wall due to VIP and stimulating histamine releasing from the mast cells, and 4) perceiving sensitivity therefore these cells are concentrated in the tip of fingers and nose.
The macrophages (dendritic cells of Langherhans) are the largest cells of the basal layer. They have a number of processes extending into the spinous layer. The macrophage nucleus sometimes consists of several lobules. The best-developed organelles are lysosomes containing cholesterin-sulphatase and others enzymes. The cell cytoplasm contains the lamellar granules. The macrophages are able to migrate into the dermis and regional lymphatic nodules. The dendritic cells of Langherhans provide: 1) producing interleukin 1 (IL-1), which stimulates proliferation and differentiation of lymphocytes, 2) engulfing antigens and representing them to lymphocytes of the epidermis and regional lymphatic nodules, 3) releasing the prostaglandin, chalons, epithelial growth factor, enzyme cholesterin-sulphatase, which splits the glue-like substance (cholesterin-sulphate) holding the superficial keratinocytes with each other, 4) forming the center of the epidermal proliferative units. Within the units the macrophages regulate the proliferation and keratosis of keratinocytes due to releasing the epithelial growth factor, chalons, and cholesterin-sulphatase.
The epidermal proliferative units are column shaped their basal part lies on the basement membrane while their apical part reaches the epithelial surface. All keratinocytes of the epidermal proliferative unit are derived from single stem cells. In the basal end of the proliferative unit the dendritic cell of Langherhans lies.
The Spinous Layer (Fig. 150 3)
Above the basal layer there are several layers of polygonal keratinocytes that constitute the spinous layer. Amongst the keratinocytes the dendritic cell processes are seen. The nuclei of cells, applying to cell basal layer, are round while the nuclei of keratinocytes, applying to the granular cell layer, are flat. The cells have processes, called spines, containing fibrils (made up of bundles of keratin filaments). The spines of one cell come in contact with spines of the neighboring cell. Between the spines there is desmosome. The cells proceed to the synthesis of the keratin, from which tonofilaments are formed. The filament bundles collect to form intermediate fibrils (tonofibrils). The spinous keratinocytes produce lamellar granules, which contain lipid substances (cholesterin-sulfates). The basal and spinous layers together are called the germinal zone of the epidermis. The external spinous epithelial cells, undergoing differentiation, migrate into the granular layer.
The Granular Layer (Fig. 150 4)
Overlying the spinous layer there are a few (3 to 4) layers of flattened cells that are characterized by the presence of deeply stained granules in their cytoplasm. These cells constitute the granular layer. The nuclei of cells in this layer are condensed and dark stained (pyknotic nuclei). Every cell of this layer proceeds to the keratin synthesis. Here the filagrin, keratohyaline, and keratolaminine are synthesized. The filagrin pack the keratin tonofibrils to form keratohyaline granules. The keratolaminine applies to the cell membrane so it becomes high dense and gains the resistance to the exposure enzymes of keratinosomes (lamellar bodies or keratin bodies) and lysosomes activated by dendritic cells of Langherhans. In granular layer cells the nuclei and organelles begin destroying and the proteins, lipids, polysaccharides, and amino acids are released and combine with keratohyaline granules. These granules are diffusely scattered in the cell cytoplasm. The formation of the keratohyaline granules is the first stage of the keratosis. In this layer cells the formation of the lamellar granules, containing cholesterin-sulphates and enzymes occurs. The keratinocytes release cholesterin-sulphates into intercellular spaces. Here the cholesterin-sulphates (glue-like substance) hold cells of granular, lucid, and cornified layers. Due to the glue-like substance the water resistant layer of the skin is formed. This layer prevents the skin dehydration and at the same time it is the barrier preventing passing the bacteria, chemical substances, and other harmful components through the skin. Quantity of desmosomes, joining the keratinocytes of the granular layer, decreases. As the keratinocytes differentiate they go upwards into next (lucid) layer.
The Lucid Layer (Fig. 150 5)
Flattened cells represent it. Their nucleus and organelles undergo destroying. Between the cells desmosomes are absent. The cells are joined with each other by glue-like substance. The keratohyaline granules fuse with one another to form the homogeneous substance called the eleidin. The eleidin formation is the second stage of the keratosis. The eleidin is not stained by dyes but it refracts light rays well therefore it shines. The lucid layer cells differentiate more and more and enter the cornified layer.
The Cornified Layer (Fig. 150 6)
This is the most superficial layer. It consists of the cornified squamous cells (flattened scales) covered by thick cell membrane. To the internal surface of the cell membrane the longitudinal keratin tonofibril bundles apply. These bundles, devoid of filagrin, and which is broken down up to the amino acids, are included in the keratin. Keratin structures of the cornified squamous cells are the soft keratin. In the cornified squamous cells instead of the nucleus the air vesicle is seen. The glue-like substance (cholesterin-sulphate), holding the superficial cornified squamous cells, is destroyed by the cholesterin-sulphatase released by dendritic cells of Langherhans. Therefore the superficial cornified squamous cells shed off from the surface of the epidermis. The cornified layers of the palms and feet are about 600 mm in thickness. This thick layer is impenetrable for the heat, water, bacteria, and toxins.
The time between the formation of the keratinocyte in the basal layer of the epidermis and its shedding off from the surface of the epidermis is about 3-4 weeks. Cell of Langhergans, keratinocytes, lamellated bodies (keratinosomes), and other cells and substances take part in process of keratosis.
The mechanical friction of the skin, the vitamin A need, the cortisol (hormone released by the adrenal cortex) excess lead to the intensity of the keratosis.
The Dermis
The dermis (Fig.
150 II) thickness is from 0.5 (the face and head) to
The papillary dermis consists of the loose connective tissue, containing the collagen, reticular and elastic fibers, running in various directions, fundamental substance, fibroblasts, macrophages, mast cells, and single smooth muscle cells (sometimes formed in bundles as arrector pili muscle).
The papillae of the
papillary layer extend into the epidermis to form the elevations and depressions
on the epidermis surface. These are most prominent on the palms and ventral
surface of the fingers and on the corresponding surface of the feet. Here the
elevation form characteristic epidermal ridges (their height is about
The reticular dermis is made up of the irregular dense connective tissue predominantly including collagen fibers orientating in various directions. The fibres interlace to form reticulum. In the places where the skin undergoes pressure or friction, the collagen fibers are thick but these places contain few elastic fibers. In the places, where the skin is stretchable, the collagen fiber bundles are thin while these places ńontain a number of elastic fibers. The reticular dermis provides the mechanical stability of the skin.
Subcutaneus Laer (Hypoderma)
The hypoderma Fig. (150 III) predominantly consists of the adipose tissue. It provides 1) motility of the skin in relation to the deep tissue, 2) decreasing the rough mechanical action on the skin, and 3) the warmth of the body.
The Skin Pigment
The pigment of the skin is called melanin. The black race person skin contains much pigment while the white race person skin contains little pigment but the skin of the person, suffering from albinism, does not contain the pigment. The epidermal melanocytes synthesize the pigment from the amino acid tyrosine. On the rough ER the tyrosine enzyme and DOPA enzyme are synthesized, which travel to the Golgi complex, where they are covered with the membrane to form vesicles containing the enzymes. The amino acid tyrosine, entering the pigment cell, combines with structures called the premelanosomae. The latter, containing amino acid tyrosine, combine with vesicles, containing the enzymes, to form the melanosomae. Within the melanosomae tyrosine enzyme influences the amino acid tyrosine to form the dihydroxy-phenylalanine. The DOPA enzyme influences the dihydroxy-phenylalanine to form the melanin. The melanin granules are released from the melanocytes and engulfed by keratinocytes. The DOPA enzyme is the marker of the actual melanocytes.
The Melanin Synthesis Regulation
The hormones of the hypophysis middle part and adrenal cortex regulate the melanin synthesis. The ultraviolet rays stimulate the activity of the tyrosine enzyme.
The Melanin Signification
The melanin protects the deep tissues from ultraviolet rays because it intakes these rays. Therefore the sunburn is useful for the young persons. The sunburn is not useful for the old persons because the ultraviolet rays provide the vitamin D accumulation in the skin. The vitamin D stimulates the calcium metabolism. In the young person the calcium deposits into the bone so the bone becomes strong. In the old man the bone contains enough calcium therefore the latter deposits into the blood vessel walls to provide their sclerosis.
The Skin Blood Supply
In the skin there are 2 arterial plexuses: 1) subpapillary arterial plexus and 2) dermal arterial plexus. From these plexuses arterioles issue. The arterioles branch to form capillary networks surrounding the fat lobules, hair roots, sweat glands, and sebaceous glands.
The capillary loops, arising from the subpapillary arterial plexus pass into each dermal papilla. The capillary loops have the ascending and descending ends. The descending end runs into venules. The venules enter the first or second subpapillary venous plexuses. The subpapillary venous plexuses give off veins reaching the deep dermal venous plexus.
The Skin Lymphatic Vessels
In the skin there are 2 lymphatic vessel plexuses: 1) subpapillary lymphatic plexus and 2) deep lymphatic plexus of skin located between the dermis and the subcutaneous layer.
The Skin Nerve Supply
The subepidermal nerve plexus, localized in hypoderma, provides the skin innervation. This plexus includes sympathetic and parasympathetic efferent nerve
fibers ending with neuromuscular terminals on the smooth muscle cells or with neurosecretory terminals on the skin glands. A number of afferent (sensitive) nerve fibers of the nerve plexus, reaching all skin layers, form many nerve plexuses surrounding the hair roots, sebaceous and sweat glands. The afferent nerve fibers end with free nerve terminals and menisci of Merkel on cells of Merkel in the epidermis, with encapsulated nerve corpuscles in the papillary dermis (tactile corpuscles), with bulbous corpuscles in the skin of the genitalia, and with lamillar corpuscles deep in the reticular dermis.
The most sensitive
parts of the skin (tip of nose or digits) contain about 300 receptors on the
square
The Sweat Glands
The sweat glands
(Fig. 151) are developed at the third month of the embryonic life from the skin
epidermis extending into sub-epithelial mesenchyme. The sweat glands are
subdivided into the eccrine sweat glands and apocrine sweat glands. The most
amounts of the sweat glands are on the palms (300 on the square
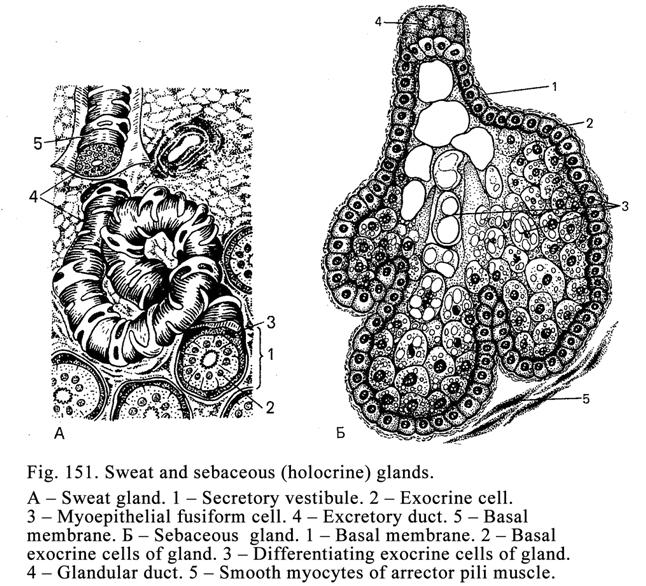
Eccrine Sweat Glands
These are simple
tubular glands. They are 30-35 mm in
diameter and
Between the basal ends of the exocrine cells and basement membrane there are branching myoepithelial cells. The myoepithelial cell processes contain contractible filaments. During the cells contract, the secretion (sweat) is released. 2 layers of cuboidal epithelial cells line the glandular duct part, running through the dermis. Within the epidermis the duct, lined by squamous epithelial cells, follows the spiral course to reach the skin surface. Its orifice is funnel shaped.
The Apocrine Sweat Glands (Fig.
The apocrine sweat glands are distincted from the eccrine sweat glands as follows. 1. They become fully developed after puberty. 2. They are connected with the sexual system function (their secretion becomes high at the time of menstruation). 3. They locate in definite places (pubis, inguinal folds, subaxillary cavities, anus, and labia majora). 4. Their terminal region is wide (150-200 mm). 5. Their excretory ducts open (along with sebaceous glands) into hair follicles. 6. They release the secretion in the apocrine way. 7. The secretion of the apocrine sweat glands is viscous and contains many proteins therefore their smell is sharp. 8. Their cell cytoplasm is stained by acid dyes. 9. The cell cytoplasm does not contain the alkaline phosphatase. The sweat glands provide 1) participation in the water and salt exchange, 2) discharge of the urea and uric acid, 3) regulation of body temperature, 4) cooling of the body in warm weather by evaporation of the sweat.
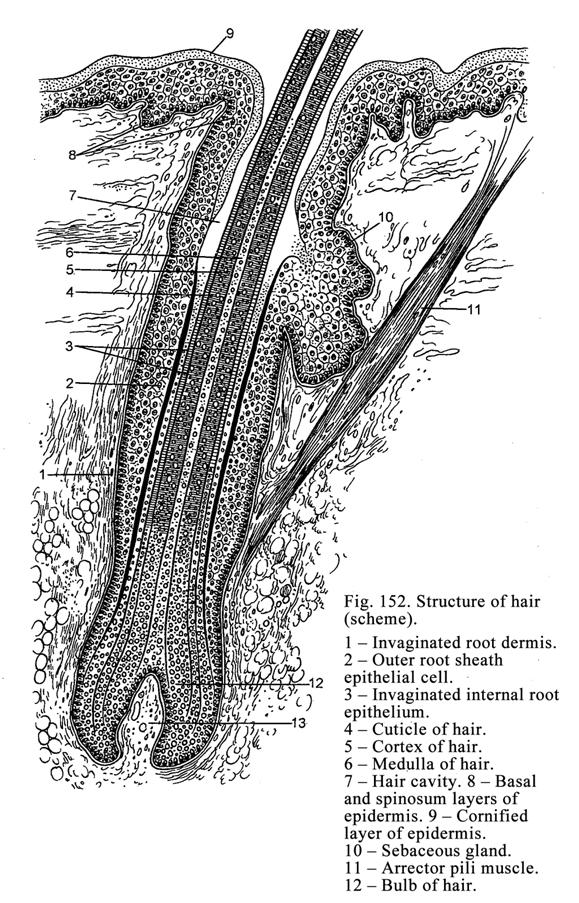
The Sebaceous Glands
The sebaceous
glands (Fig. 151 and 152 10) associate with the hair roots except the labia
minora and nipple of the breast. These glands are fully developed after the
puberty. They are simple alveolar glands. These are examples of the holocrine
glands. The gland terminal regions (they are 0.2-
Every day the
sebaceous gland discharge about
Hairs
Hairs cover the
entire skin except palms, soles, ventral surface and sides of digits, and some
parts of the male and female external genitalia. These are divided into long
hairs (head, beard, moustache, subaxillary cavities, and pubis), bristle hairs
(eyelash, brows, external acoustic meatus, vestibule of the nasal cavity). The
rest hairs are called lanugo. Hairs are 0.05 to
The Hair Development
The hairs develop at the third month of the embryonic life. The epithelial cords extend from the epidermis into subjacent mesenchyme. The cord ends are dilated to form the hair bulb. The connective tissue, extending into the bulb, is called the papilla of the hair follicle. Capillary vessels of the papilla provide the bulb blood supply. The bulb is the matrix of the hair root. Due to the proliferation of the bulb cells the hair root grows.
The Hair Structure
The hair root (Fig. 152) ends with the bulb. The follicle, consisting of the internal root epithelium and the outer root sheath epithelial cells, surrounds the root. The invaginated root dermis surrounds the root sheath epithelial cells.
The medulla of the hair is located in its center. The medulla cells are derived from the bulb proliferating cells. Near the bulb the flattened medulla cells, containing flat nuclei, are seen. Their cell cytoplasm contains keratin filaments undergoing the keratosis so the filaments convert into trichohyaline granules. Above sebaceous gland duct entering the trichohyaline granules undergo keratosis to form soft keratin within the medulla cells so the latter convert into polyhedral keratin scales, losing nuclei, containing the pigment and air vesicles.
The cortex hair cells are derived from bulb cells. Near the bulb the prismatic cortex cells direct perpendicularly in relation to the hair shaft. Soon the cells lose nuclei and convert into keratin scales containing hard keratin, pigment and air vesicles. In an old person amount of the pigment decreases but the number of air vesicles increases so the hairs become grey.
The hair cuticle is derived from the bulb cells. Near the bulb the cuticle cells are column shaped directing perpendicularly in relation to the hair length. In distant cuticle cells lose nuclei and are converted into keratin scales lying in the slope position. The scales contain hard keratin but pigment granules are absent.
The internal root epithelium of the follicle is derived from bulb cells. Near the bulb the internal root epithelium consists of 3 layers: 1) cuticle, 2) internal (granuliferous) epithelial layer, and 3) external (pale) epithelial layer. In the middle part of the hair root three layers fuse to form one layer. On the sebaceous duct level the internal root epithelium disappears.
The invaginated external root epithelium of the root follicle is the epidermis germinal layer (germinal zone) surrounding the internal root epithelium. Near the bulb the former becomes thin. When the epidermis is damaged, the invaginated root epithelium takes part in its regeneration.
The invaginated root dermis consists of the internal circular layer of the collagen fibers and external longitudinal layer.
The Arrector pili muscle internal end is connected with the invaginated root dermis while its external end is connected with papillary dermis collagen fibers. The arrector pili muscle is only in the head hairs. In the angle between the hair root and arrector pili muscle there is the sebaceous gland terminal region. When the arrector pili muscle contracts, the angle alters and as a result the sebaceous gland releases the sebum. The latter covers the epidermis. It decreases the water vaporization from the skin surface and keeps the body warmth
The Change of Hairs
The hairs change in 2-5 years and this process consists of 3 phases. The first phase lasts about 2 weeks. It is characterized by the atrophy of the papilla of hair follicle and cessation the multiplication of the bulb cells. These cells undergo keratosis. The end of the hair assumes a bulb-like appearance, which separates from the papilla and moves upwards. On the level, where the arrector pili muscle attaches, the movement of the bulb-like hair stops. The internal root epithelium is destroyed; the invaginated external root epithelium is kept (preserved).
In the second phase the bulb-like hair does not alter during the 2-4th month. In the third phase the invaginated external root epithelium stem cells begin dividing and as a result the new bulb is formed, into which the new papilla extends. Due to the multiplication of the new bulb cells the new hair and new internal root epithelium begin growing. The new growing hair pushes the existing bulb-like hair.
The Nail
The nail is a cornified layer of the epidermis consisting of the keratin.
The Nail Development
The nail begins
developing at the third month of the embryonic life. The finger ending phalanx
epidermis becomes thick and immerses into the subjacent connective tissue so
the nail bud is formed. From the bud proximal end the nail begins growing. The
growth velocity is 0.25-
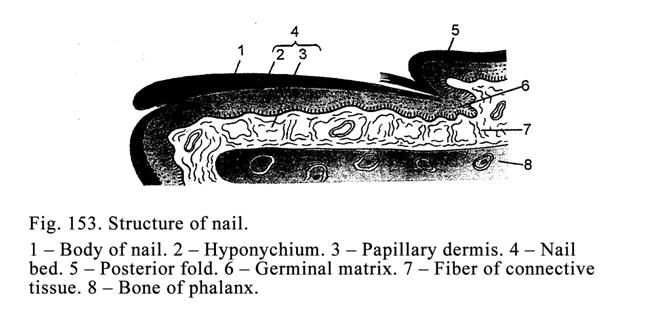
The Nail Structure
The nail (Fig. 153) lies on the nail bed consisting of the epidermis germinal layer, called the hyponychium, and connective tissue. At the base and sides of the nail bed is limited by folds. Between the folds and the nail bed there are fissures occupied by the side margins and the root of the nail. The epidermis, which is on the external surface of the nail, is called the eponychium.
The nail bed proximal part is the matrix, from which the nail grows. The matrix multiplication cells undergo keratosis to form cornified scales applying to the proximal end (root) of the nail so the latter grows in length. Below the nail bed germinal layer the connective tissue lies, in which the longitudinal and perpendicular collagen fibers are present. The perpendicular fibers extend into the phalanx periosteum. In the nail bed connective tissue the blood vessels are seen.
The nail consists of the closely applying to each other cornified scales. In the nail there is the root, the body, and the margin. In the thumb the nail root part is seen. It is called the lunula.
The Skin Functions
1. The skin provides the mechanical, physical, and biological protection of the body. 2. It prevents passing of the water and solved in it substances. 3. The skin takes part in the excretion of the water and salts. 4. The sweat glands release the urea, uric acid, and others substances. 5. The skin regulates the body temperature. 6. Some degree of exposure to sunlight is essential for synthesis of vitamin D. 7. The blood accumulates in the skin blood vessels. 8. The skin takes part in the immune protection of the body. 9. The skin keratinocytes synthesize the thymosin and thymopoietin stimulating the antigen nondependent differentiation of the T-lymphocytes. 10. The skin is receptor organ (the skin receptors are sensitive to the touch, temperature, pain, and pressure).
CHAPTER
25
URINARY
ORGANS
The urinary organs include kidneys and urinary tracts (calyces, pelvises, ureters, urinary bladder, and urethra).
The Kidneys
They produce the urine. Many harmful wastes are removed from blood trough kidneys.
The Kidney Development
The parenchyma of kidneys (glomerular capsule, renal tubules, and collecting ducts) is derived from mesoderm. The kidney stroma (interstitial tissue) and smooth muscle are derived from the mesenchyme.
The Kidney Structure
The peritoneum covers the kidney front surface. This organ is bean shaped. The connective tissue capsule covers the kidney. The subcapsular mass is called the renal cortex. Its thickness is about half of the kidney thickness. The internal part of the kidney is called the medulla dividing into the external zone, applying to the cortex, and internal zone. The renal cortex is deep red while the renal medulla, consisting of 8-12 renal pyramids, has lighter colour. The pyramid apices direct towards the calyxes but the wide pyramid bases apply to the renal cortex. The renal columns extend into the renal medulla. The medullary rays, consisting of collecting ducts, extend into renal cortex.
The kidney consists of renal lobes and lobules. Every lobule includes the renal pyramid and part of cortex opposite the pyramid base. The kidney stroma is made up of loose collagen connective tissue containing reticular fibers and reticular cells. The kidney parenchyma consists of nephrons.
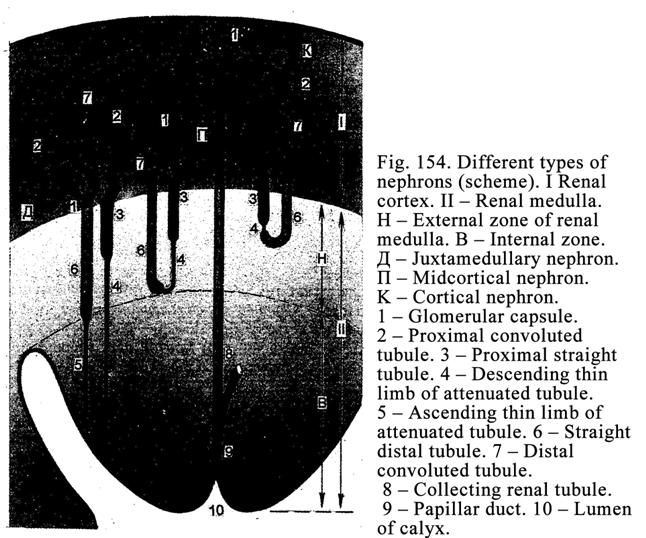
The Nephron
The nephron is a structural and functional unit of the kidney. It includes the glomerular capsule and renal tubules. The tubules are the proximal convoluted tubule, proximal straight tubule, attenuated (descending thin limb of nephron loop) tubule, straight distal tubule (ascending thick limb of nephron loop), and distal convoluted tubule.
Classification of the Nephrons
In the kidney cortex there are 3 nephron types: 1) cortical nephrons constituting about 15% localizing in the renal cortex (Fig. 154K), 2) midcortical nephrons (Fig. 154Ļ) forming about 70% (their convoluted proximal and distal tubules and renal corpuscles lie in the renal cortex while the descending thin limbs, running into the straight distal tubules, reach the external medullary zone), and 3) juxtamedullary nephrons (Fig.154Ä) constituting about 15% (their convoluted proximal and distal tubules and renal corpuscles locate in the renal cortex [Fig. 155 & Fig. 156I] near the renal medulla while their thin limb of renal loop reach the internal medullary zone).
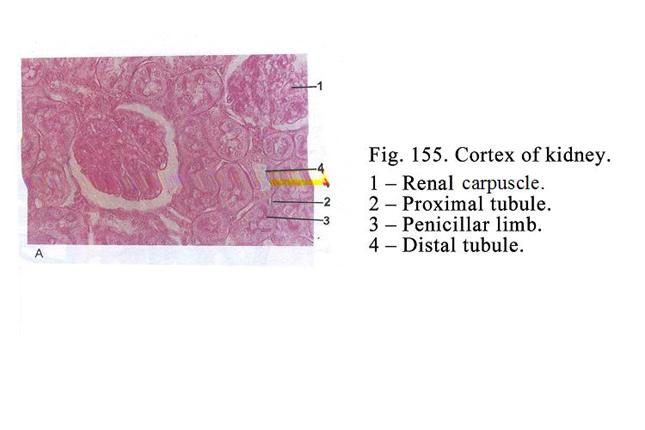
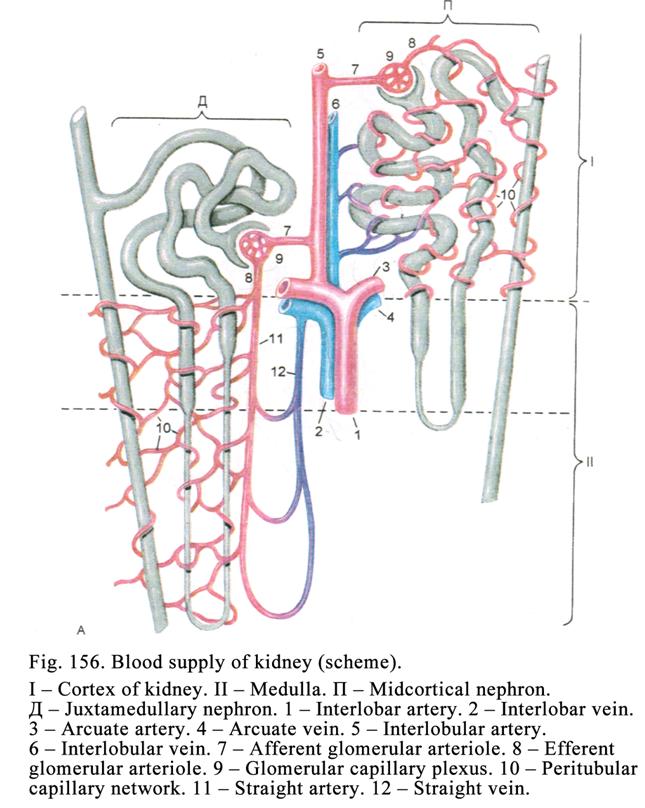
Renal Blood Vessels
At the hilum of the kidney renal artery divides into interlobar arteries reaching the level of the pyramid bases and dividing into the arcuate arteries, from which the interlobular arteries issue running along the boundary between the lobules. The interlobular arteries give off intralobular arteries (Fig. 156).
In case the intralobular arteries reach the midcortical nephron corpuscles, these arteries belong to the cortical blood vessel system. In case intralobular arteries reach the juxtamedullary nephron corpuscles, they belong to the juxtamedullary circulation.
The Kidney Cortical Circulation (Fig. 156Ļ)
The afferent arteriole, issuing from the intralobular artery, approaches the midcortical renal corpuscle. This arteriole branches to form glomerulus (glomerular capillary network) located within the glomerular capsule. The capillaries of the capillary network drain into the efferent arteriole (the glomerular capillary vessels are between the afferent and efferent arterioles).
The afferent
arteriole diameter is more than that of the efferent arteriole therefore the
pressure in the glomerular capillary vessels is higher than
Due to the glomerular blood capillary vessel high pressure the midcortical nephrons provide the urine formation.
The Kidney Juxtamedullary Circulation
(Fig. 156Ä)
The intralobular artery afferent arterioles reach the juxtamedullary renal corpuscles. The diameter of the afferent arterioles is less than that of the efferent arterioles so the glomerular blood capillary vessel pressure is low, therefore the urine formation is limited in juxtamedullary nephrons.
The efferent arterioles are partly branched into the capillaries surrounding the straight renal tubules partly give off the recta blood vessels (vasa recta, running towards the renal medulla). On the various levels the vasa recta form loops running into vein recta. The latter return to the boundary between medulla and cortex and run into the arcuate vein. It is seen that the oxygenated blood flows through the loop descending limb to the medulla through the loop ascending this blood flows towards the cortex. It is called the countercurrent blood flow system.
From the vasa recta
the capillary vessels issue. These capillaries surround the straight tubules
(proximal and distal straight tubules descending and ascending limb of loop)
and run into vein recta.
The Juxtamedullary Circulation
Signification
Due to the low intra capillary pressure in glomerular capillary plexus the urine formation is limited. The juxtamedullary circulation plays the role of the shunt i.e., it facilitates the blood flow from arteries to veins.
The Urine Formation
The urine formation includes 2 phases: 1) filtration phase and 2) reabsorption phase.
The Filtration Barrier Providing
Filtration Phase
The filtration phase, provided by the glomerular filtration barrier, occurs in the renal corpuscle (157). The latter consists of the glomerulus, including about 50 blood capillary vessel loops, lined with the fenestrated endothelial cells (endothelium), and glomerular capsule. The endothelium lies on the basement membrane including 3 laminae.
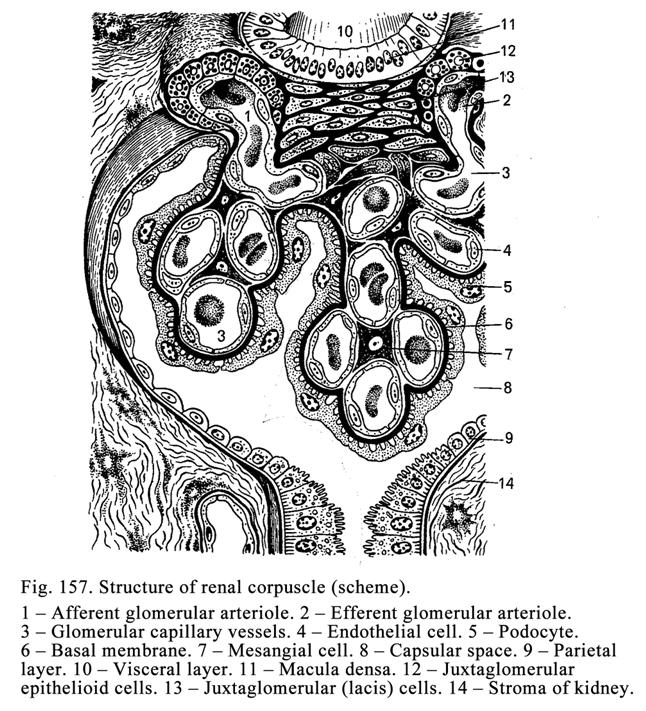
The glomerular capsule consists of 2 layers: 1) parietal layer and 2) visceral layer. The parietal layer is lined with the simple squamous or cuboidal epithelium, which is the continuation of that of the proximal convoluted tubule.
The visceral layer structure is more complicated than that of the parietal layer. It forms deep folds surrounding almost every capillary loop of the glomerulus. The place, where the visceral layer does not extend, the mesangium occupies. The mesangium consists of the mesangial matrix and mesangial cells (macrophages, monocytes, and muscle-like cells). The muscle-like cells release the mesangium matrix components and are able to contract. The macrophages provide the protective function and belong to the kidney immune system. The flattened epithelial cells, called podocytes (Fig. 158), line the capsule visceral layer.
Every podocyte contains the invaginated nucleus. Large processes, called the primary plate or trabeculae issue from the podocyte body. From the primary plate small processes issue, called the secondary processes. The secondary processes rest on the same membrane, to which opposite surface the fenestrated endothelial cells of glomerular capillary vessels apply. The podocyte cell membrane bears receptors perceiving immunoglobulins and complimentary proteins. It means that podocytes take part in immune reactions. The podocyte rough ER produces the basal membrane components, substances, regulating the blood flow in glomerular capillary vessels, and factors inhibiting proliferation of the mesangial cells.
Between the podocyte secondary processes, resting on the basement membrane, there are splits. The splits direct towards the boundary between the adjacent podocytes. Here the splits are communicated with the glomerular capsule space. The splits, located between the secondary processes are closed by basement membrane.
The
basal membrane is made up of three layers. In the
membrane there is the central electron dense layer (lamina densa) and outer and
inner lucent layers (laminarara externa and interna). The lamina densa contains
the network of collagen fibrils. The loops of the network are 4-7 nm in
diameter. Through these small loops the small substances can pass (their
diameter must be less than 7 nm). Therefore through the basal membrane the
small molecules of the proteins, electrolytes, and water can pass but the large
molecules of the proteins, and blood cells cannot pass. The basal membrane
contains proteoglycans, with negative charge, which increases from the internal
membrane surface to the external one and to the podocytes. Thus, in the renal
corpuscle there is the glomerular filtrating barrier including 3 components: 1)
fenestrated endothelial cells of glomerular capillary vessels, 2) basal membrane,
and 3) podocytes of the visceral layer. Through the barrier the following
components of the blood plasma can pass: water, small molecular proteins,
electrolytes, harmful waste products (that result from metabolism), and
glucose. All these filtrated components constitute the primary urine. Every day
more than
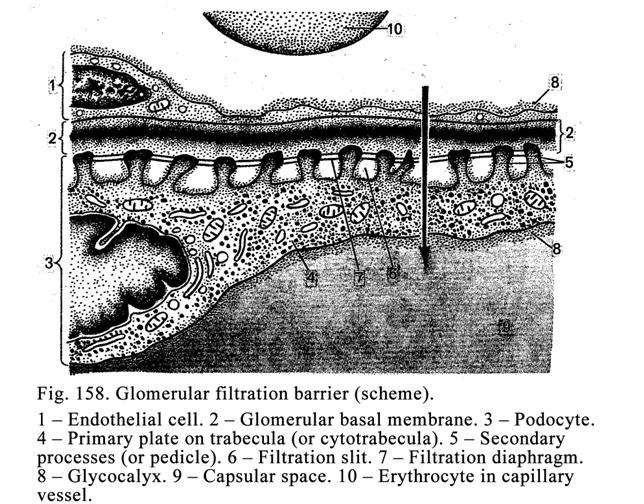
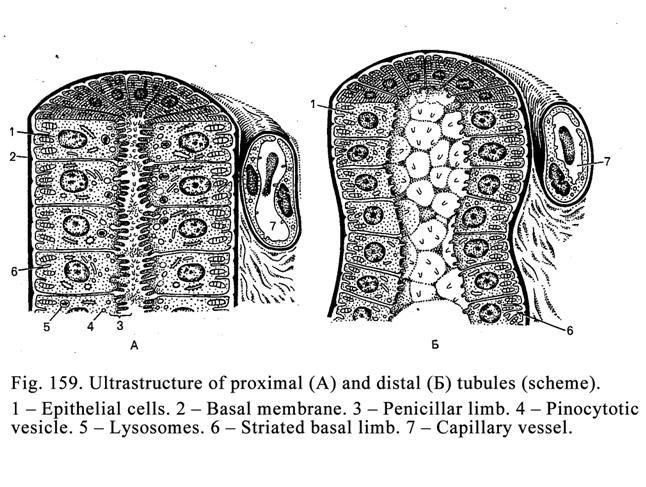
The Reabsorption Phase
The reabsorption
phase occurs in renal tubules. Every kidney contains about 1 million of
nephrons. The length of tubules of every nephron is about
The proximal (Fig. 159A) part consists of the proximal convoluted tubule and proximal straight tubule. It is about 60 mm in diameter and lined with simple cuboidal epithelium. Its epithelial cells contain a round active nucleus and a muddy cytoplasm. The epithelial cell apical part bears the penicillar limb (consisting of microvilli) on the cell basal part there is the striated basal limb (cell membrane folds occupied by mitochondria). In the cell cytoplasm there are lysosomes, pinocytotic vesicles, and mitochondria containing mitochondrion enzymes.
In the proximal tubules (convoluted and straight) the following substances are reabsorbed: 1) glucose (entire quantity of the glucose is reabsorbed), 2) proteins (small protein molecules are reabsorbed), 3) part of water, and 4) part of electrolytes.
The proteins, passing into epithelial cells, broken by lysosome enzymes into amino acids, migrate into the blood capillary vessels and are carried with blood to various organs. In these organs the amino acids undergo the synthesis to form other (new) proteins. Thus, in the kidneys not only the urine is formed but also they take part in the body protein renewing.
The electrolytes undergo reabsorption due to the mitochondrion enzymes (special electrolyte enzymes: of Na+ ATPase, K+ ATPase, and Ca++ ATPase) and striated basal limb. The rest urine runs into the attenuated tubule.
The attenuated tubule (Fig. 160) is 13-15 mm in diameter. The flattened epithelial cells, containing poorly developed organelles and pale cytoplasm, line the attenuated tubules. In the attenuated tubule the water only undergoes reabsorption. From the attenuated tubule (descending thin limb) the rest urine runs into the straight distal tubule (ascending limb), which continues with the convoluted distal tubule. The distal straight and convoluted tubules constitute the distal part of the nephron.
Distal tubules (straight and convoluted) are 20-50 mm in diameter. Light cuboidal epithelial cells, containing round active nuclei, line the distal tubules. On the apical part of the epithelial cells the penicillar limb is absent while on the basal part the striated basal limb (Fig. 159 Į) is seen. The limb contains electrolyte ATP enzymes and mitochondrion enzymes. The cell cytoplasm contains special enzyme kallikrein.
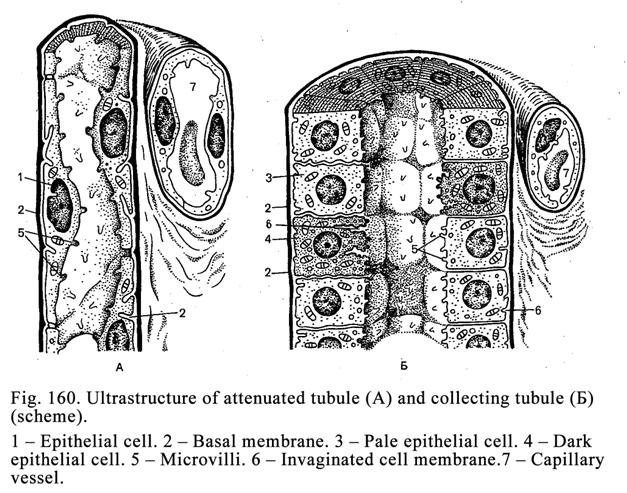
In the straight distal tubule and applying half of the convoluted distal tubule the electrolytes are only reabsorbed. Therefore the electrolytes accumulate in the renal stroma round the renal tubules and provide the high osmotic pressure here. In the primary urine, flowing through this distal part of tubule and losing electrolytes, the osmotic pressure becomes low. Therefore when the urine flows into the second half of the distal convoluted tubule the water passes through the tubule wall into surrounding connective tissue. This is called the facultative reabsorption. The rest urine, containing a number of electrolytes and harmful waste products, flows into the collecting renal tubule (Fig. 160 Į).
The cuboidal epithelial cells line the cortical part of collecting renal tubule while the columnar epithelial cells line its medullary part. In the collecting renal tubule wall there are 2 types of epithelial cells: 1) dark cells releasing the hydrochloric acid, and 2) light (lucid) cells reabsorbing water and secreting prostaglandin. The reabsorption of the water through the wall of the collecting renal tubules and applying parts of the distal convoluted tubule is regulated by vasopressin produced by the hypothalamus. If the vasopressin is absent, the water is not reabsorbed from the distal parts of distal convoluted tubules and collecting tubules. From the collecting tubules the urine flows into papillary ducts, from it into calyces, from here into pelvis, from the latter into ureter, then into urinary bladder, and at last into urethra. The collecting tubule dark cells release the hydrochloric acid and the urine becomes acidulated (it is the third phase of the urine formation).
The Kidney Endocrine System
It includes 3 apparatuses: 1) juxtaglomerular complex, 2) prostaglandin apparatus, and 3) kallikrein kinin apparatus.
The Juxtaglomerular Complex (Fig. 157 11,
12, 13)
The complex includes: 1) juxtaglomerular epithelioid cells, 2) macula densa, and 3) lacis cells.
The juxtaglomerular epithelioid cells are located in the wall of the afferent and efferent arterioles (Fig. 157 12). The cells are cub shaped and contain the light cytoplasm, synthetic apparatus (Golgi complex, ER, and mitochondria), and secretory granules. The juxtaglomerular epithelioid cells release renin, stimulating the blood pressure increase and releasing of the aldosterone from the adrenal cortex glomerular zone. The aldosterone stimulates the reabsorption of the sodium and chlorine through the wall of distal tubules.
The macula densa (Fig.157 11) locates in the wall of the distal tubules near the glomerulus between the afferent and efferent arterioles. The macula densa cells are thin and tall they rest on the thin basal membrane. The macula densa cells are sensitive to the sodium in urine flowing through the distal tubules. If the urine contains much sodium, these cells influence the juxtaglomerular cells, which release the renin. The latter stimulates the blood vessel musculature contraction (through angiotensin I and II) and aldosterone releasing. As a result the blood pressure and the pressure of glomerular capillary plexus become high. It stimulates the blood plasma components filtration from the glomerular capillary vessels into the glomerular capsule and also stimulates the sodium reabsorption (the aldosterone stimulates the sodium reabsorption) from the primary urine into the capillary vessels surrounding the renal tubules. It leads to the decrease of the sodium contents in the primary urine and to the stoppage of affecting of the macula densa and influencing the latter the juxtaglomerular cells so these cells release less renin.
The lacis cells (Fig. 157 13) localize in the interval between the macula densa and the afferent and efferent arterioles. The lacis cell long processes come in contact with the mesangial cells. The lacis cells and mesangial cells probably produce the renin, if the juxtaglomerular cells stop releasing this hormone.
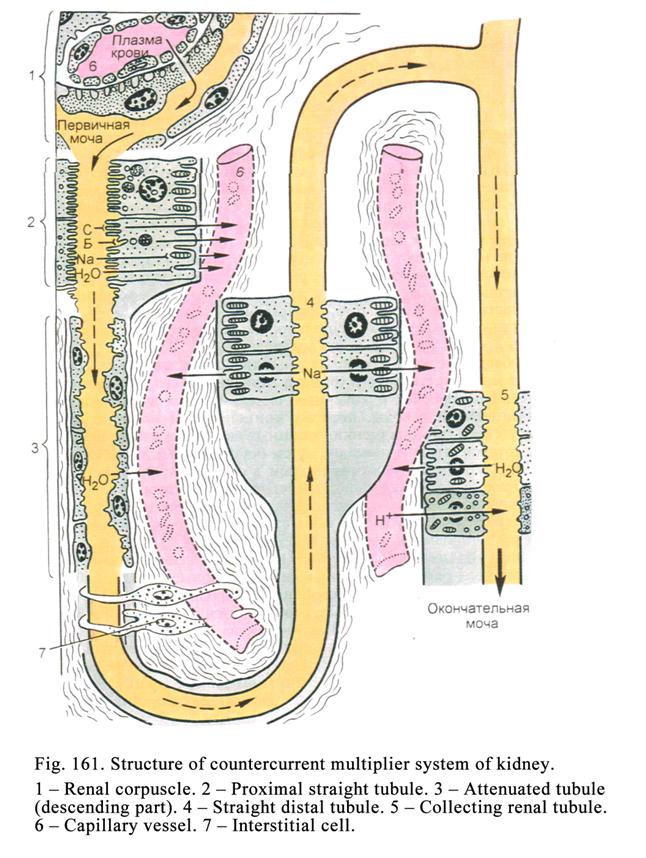
The Prostaglandin Apparatus
It consists of the interstitial cells (Fig. 161 7) of renal medulla and the clear cells of the collecting duct wall releasing the prostaglandin.
Interstitial cells are spindle shaped their body gives off processes. Some processes come in contact with blood capillary vessels others come in contact with straight tubules (straight distal tubules, straight proximal tubules, descending thin limb of nephron loop and its ascending limb). In the cells there are Golgi complex, ER, mitochondria, and prostaglandin granules. The prostaglandin provides lowering of the blood pressure and sodium reabsorption from the renal tubules and the urine contains more sodium.
The Kallikrein Kinin Apparatus
It consists of some distal tubule epithelial cells. From the blood plasma to the epithelial cells the kinin precursors pass. The cell kallikrein influence the precursors to form kinin. The kinin activates the prostaglandin secretion from the prostaglandin apparatus cells so the blood pressure and reabsorption of the sodium from the renal tubules decrease. It leads to the increase of the sodium in the urine and the urine volume.
The Kidney Lymphatic Vessels
The lymphatic vessels of the kidney include the lymphatic capillary network, from which the lymph runs into the primary, secondary, tertiary, and quaternary lymphatic vessels and interlobar sinuses, from which the lymph flows into the regional lymphatic nodes.
The Kidney Nerve Supply
The efferent (sympathetic and parasympathetic) nerve fibers and afferent (sensitive) nerve fibers innervate the kidneys. The efferent nerve fibers end on the blood vessels and epithelial cells of nephrons.
The Age Dependent Changes of the Kidneys
The renal cortex of the newborns is ¼-1/5 of the renal thickness; that of the adult is 1/2 1/3 part because in the growing organism the length and thickness of the renal tubules grow. The renal tubules of the newborn are 18-36 mm in diameter while those in the adult are 40-60 mm. The most growth is noted by 2 years. After it the growth is slowly prolonged up to the puberty. With growth of the body and kidney the quantity of the renal corpuscles per the volume unit becomes less. For example, per the volume unit there are 50 corpuscles in the newborn kidney, about 20 corpuscles in the one-year-old child, and about 5 corpuscles in the adult. In old persons the kidney blood vessels undergo sclerosis and in the glomerular capillary vessel pressure becomes low. It leads to low filtration of the blood plasma components from glomerular capillary vessels into the space of the glomerular capsule so the urine formation decreases. In this case the juxtaglomerular cells release much renin and the blood pressure becomes high (renal hypertonia) and the glomerular capillary pressure becomes also high. It provides the normal urine formation.
The Kidney Functions
The kidneys provide 1) the urine formation, 2) regulating of the water and salts balance, 3) regulating of the acid and alkaline balance, 4) discharging harmful waste from the organism, 5) stabilization of the organism homeostasis, 6) releasing hormones regulating the urine formation. In addition the kidney endocrine apparatus releases erythropoietin stimulating the erythrocytopoiesis.
The Urine Passages
They include the calyces, renal pelvises, ureters, urinary bladder, and urethra. The urine passage walls are made up of 4 layers.
The wall of the calyces and pelvis consists of 4 tunics: 1) mucosal tunic, 2) submucosal tunic, 3) muscular tunic, and 4) adventitial tunic. The mucosal tunic is made up of transitional epithelium and proper mucous lamina. The submucosal layer consists of the collagen loose connective tissue. The muscle tunic includes the smooth muscle bundles directing spirally. The adventitial tunic is made up of the collagen loose connective tissue.
The Ureter
The ureter wall consists of 4 tunics: mucosal tunic, submucosal tunic, muscular tunic, and adventitial tunic. The mucosal tunic, forming 10-12 longitudinal folds, includes the transitional epithelium and proper mucosal lamina. The collagen loose connective tissue of mucosal tunic continues with that of the submucosal tunic without any clear boundary.
In the submucosal tunic there are terminal regions of the ureter glands. Their ducts open on the mucosal tunic surface.
The muscular tunic in its upper part consists of the internal longitudinal muscle layer and external circular muscle layer. The lover part of the muscular tunic includes the internal and external longitudinal muscular layers and the middle circular muscular layer. The adventitial tunic consists of the collagen loose connective tissue.
The Ureter Segments and Sphincters
The ureter is divided into 3 (sometimes into 4) segments: upper, middle, and lower one. Between the segments there are sphincters. The sphincters are made up of the circular spongy blood vessels. When the vessels fill with blood, the sphincters are closed. When sphincters open, the urine flows towards the urinary bladder.
The Urinary Bladder
The urinary bladder wall (Fig. 162) includes the mucosal tunic, submucosal tunic, muscular tunic, and serous tunic (in some places there is adventitial tunic).
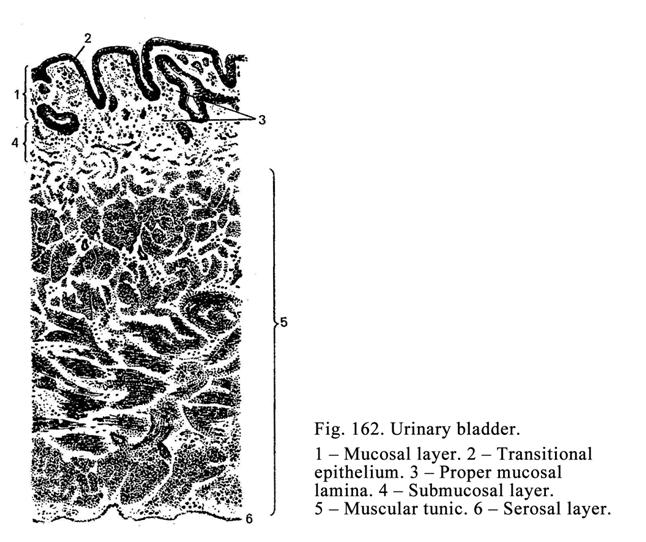
The mucosal tunic of the empty urinary bladder forms folds. The folds are sometimes similar to the tongue papillae; at the exams some students confuse the urinary bladder preparation with that of the tongue. The bladder mucosal tunic consists of the transitional epithelium and proper mucosal lamina. In the triangle between the ureters and urethra the folds of mucosal tunic are absent. Here in the proper mucosal lamina there are small mucous glands. In the triangle the submucosal tunic is absent.
The urinary bladder submucosal tunic consists of the collagen loose connective tissue.
The muscular tunic includes the internal longitudinal layer, middle circular layer, and external longitudinal. From the middle layer the urinary sphincter is formed.
The serous tunic covers the urinary bladder fundus, dorsal surface, and upper part of the bladder sides the rest bladder surface covered with adventitial tunic consisting of collagen loose connective tissue.
The Female Urethra
The female urethra includes 3 parts: upper part, middle and lower parts. The upper part is lined with the transitional epithelium, middle part is lined with the pseudostratified epithelium, and lower part is lined with the stratified squamous non-keratinized epithelium.
The Urinary System Functions
The urinary system provides 1) releasing of harmful waste products from the organism, 2) participating in the water and salt exchange, 3) regulation of the acid and alkaline balance, 4) regulating the homeostatic status, and 5) endocrine function.
CHAPTER
26
MALE
REPRODUCTIVE ORGANS
The male genitalia include the testis, seminiferous tracts (straight seminiferous tubules, efferent ducts of testis, epididymis duct, defferent duct, and ejaculatory duct running into the urethra) and accessory organs (prostate, seminal vesicles, bulbourethral glands, and penis).
Development of Male Rproductive Organs
The epithelium lining of the seminiferous tracts and sustaining epithelial cells (Sertoli cells) are derived from the mesoderm. Gametocytes are derived from the mesenchyme cells of the yolk sac wall. The connective tissue, interstitial cells, and smooth muscle of the genitalia are derived from the mesenchyme cells.
The Testis
The serous coat
covers the testis. The subjacent layer is the tunica albuginea consisting of
the dense connective tissue. The testis tunica albuginea dorsal part becomes
thick to form the mediastinum. From the mediastinum number of the septa issue
outgrow, dividing the testis (Fig. 163) into 250 lobules. In every lobule there
are 1-4 convoluted seminiferous tubules. All tubules are 350-400. When
stretched out each tubule is 30-
Within the seminiferous convoluted tubules there is the spermatogenic epithelium layer consisting of 2 cell types: sustaining epithelial cells and 2) maturing spermatogenic cells, which are derived from gametocytes.
Amongst the maturing spermatogenic cells there are 1) spermatogonia resting on basal membrane, 2) primary and secondary spermatocytes locating in the second layer, 3) spermatids localing in the third layer, and 4) spermatozoa locating in the seminiferous convoluted tubule center.
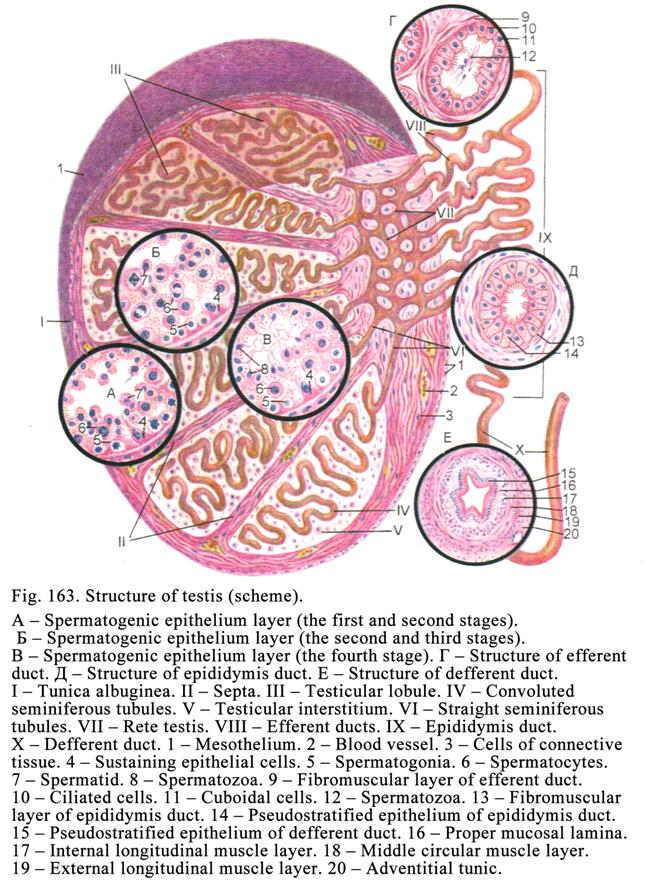
The tubule wall consists of 3 layers: 1) the basal layer, 2) the myoid layer, and 3) the fibrous layer.
The basal layer consisting of collagen fibers locate deep to the basal membrane, on which the spermatogenic epithelial layer rests.
The myoid layer is made up of the myoid cells, which ate similar to smooth muscle cells. Due to the contraction of these cells the seminiferous convoluted tubules constrict.
The fibrous layer consists of 2 parts: 1) the internal part represented by collagen fibers and 2) by the fibroblast-like cells.
The innermost
basal membrane of the seminiferous convoluted
tubule wall is thick (about 80 nm). Between the basal layer and myoid layer the
second basal membrane is seen. Between the myoid layer and the fibrous
layer there is the third basal membrane.
The sustaining epithelial cells are large. They are pyramidal shaped. Their wide basal part rests on the basal membrane their narrow apical part reaches the tubule center. On their side surface there are sockets, in which spermatogenic cells maturate. The cell membrane of the sustaining cells bears receptors for the follicle-stimulating hormone of the hypophysis.
In the seminiferous convoluted tubules there are 2 types of the sustaining epithelial cells: the dark cells and light cells. On the cell side surface near their basal parts there is outgrowth coming in contact with those of the adjacent cells to form the intercellular junction complex dividing the tubule lumen into 1) deep abluminal compartment containing spermatogonia and 2) adluminal compartment containing primary and secondary spermatocytes, spermatids, and spermatozoa. The cell oval nucleus, having light nucleoplasm and deep staining three-lobed nucleoli (near the nucleoli there are juxta-nucleolus chromatin clusters) localizes in the basal part of the sustaining epithelial cell. In the cell cytoplasm there are well developed Golgi complex, ER, mitochondria and lysosomes. The cells contain incorporations of the proteins, glycogen, and lipids.
The sustaining epithelial cells provide many functions. 1. They release the fluid secretion filling the tubule lumen (exocrine function). 2. These cells provide endocrine function (the light cells release hormone, inhibiting the secretion of the follicle stimulating hormone of the hypophysis, the dark cells release the factor stimulating the spermatogenic cell divisions and producing testosterone binding protein, which transports the testosterone from deep abluminal compartment into adluminal compartment). 3. The cells provide the nutritious function. 4. They provide the protective function (the cells provide the optimum environment for the spermatogenic cell maturation). 5. The sustaining epithelial cells engulf dead cells, their fragments, and others harmful substances.
The blood-testis barrier includes 2 parts: 1) the barrier between the blood capillary vessels and basal compartment of seminiferous convoluted tubules and 2) barrier between blood capillary vessels and adluminal compartment. The first barrier includes 1) the capillary vessel endothelium, 2) the capillary vessel basal membrane, and 3) the seminiferous convoluted tubule wall. The second barrier includes 1) the capillary vessel endothelium, 2) the capillary vessel basal membrane, 3) the seminiferous convoluted tubule wall, and 4) the sustaining epithelial cells preventing passage of the harmful substances into the adluminal compartment. If the foreign cell or own (of the host body) cell enters the seminiferous convoluted tubule, the immune reaction begins because these both cells are antigens for spermatogenic cells.
The blood capillary vessels do not have fenestrated endothelial cells because the testis hormones run into lymphatic capillary vessels.
The testis provides 1) the spermatogenesis function and 2) the endocrine function.
The Spermatogenesis
It includes 4
stages: 1) multiplication, 2) growth, 3) maturation, and 4) spermatozoon
formation (spermiogenesis).
The Multiplication Stage
At the time of the stage spermatogonia undergo the mitotic division. Amongst the spermatogonia there are stem A type dark cells (reserve non-dividing cells) and semi-stem A type light cells (rapidly dividing cells, within their nuclei there are euchromatin and prominent nucleoli). The A type light cells undergo division and the differentiating cells (spermatogonia) are formed (A type and B type). The B type differentiating cells contain a larger nucleus and more rough chromatin clusters than the A type differentiating cells.
In the process of the differentiating cell division the cell chain is formed (the cells connect with each other by cytoplasm processes). Every cell nucleus of the chain contains 46 maternal chromosomes while the every maternal chromosome includes 2 daughter chromosomes (chromatids) i.e., each cell nucleus contains 92 chromatids.
At the same time the intercellular junction complexes between the adjoining sustaining epithelial cells open and chains of the differentiating spermatogonia pass from basal (abluminal) into adluminal compartment. After passing the growth the second stage begins and the cells are called primary spermatocytes.
The Growth Stage
The growth stage includes 5 phases: 1) leptotene, 2) zygotene, 3) pachitene, 4) diplotene, and 5) diakinesis.
Leptotene is characterized by the chromosomes undergoing coiling so they are seen as threads.
Zygotene is characterized by the chromosomes united with each other to form pairs or bivalents. Every bivalent (or tetrad) includes two central and two peripheral chromatid. At the same time the central chromatids of the bivalent exchange genes (crossing over) so the genetic material of each of chromatid (monad) of the bivalent (tetrad) has distinct genetic contents.
Pachitene is characterized by further coiling of chromosomes so they become thicker and shorter.
At the time of diplotene two chromosomes (dyads) of bivalent (tetrad) now try to move apart and between the monads of the dyads the slit is seen. The dyads connect only with each other at the points of crossing over.
Diakinesis is characterized by further coiling of the monads and dyads of the tetrads.
Thus, from every bivalent one tetrad is formed every tetrad consists of two dyads and four monads. Each nucleus of the primary spermatocyte includes 23 tetrads or 46 dyads or 92 monads.
The Maturation Stage
This stage includes 2 meiotic divisions (the first and second divisions).
The first division begins after the formation of tetrads. The tetrads are in equator of the primary spermatocyte so that one half of the tetrad (dyad) directs towards the one pole another half towards the second pole. After it dyads move to the poles of the cell (anaphase). After telophase two cells, called the secondary spermatocytes, are formed. The nucleus of the secondary spermatocyte contains 23 dyads (46 monads).
The second division begins soon after the first division. Dyads are in the cell equator so that one monad directs towards the one pole other monad towards the second pole. At time of anaphase the monads disperse to different poles. Then telophase begins. After the telophase 2 spermatids, containing 23 monads (chromosomes), are formed.
Spermiogenesis
At the time of spermiogenesis the spermatids (small round cells with spherical nuclei) immerse into the sustaining epithelial cells sockets. On the spermatid nucleus pole, directed to the sustaining epithelial cell, Golgi complex localizes on the opposite nucleus pole the diplosome, consisting of 2 centrioles (proximal and distal), localizes.
The Golgi complex is transformed to form dense granule, which grows and covers the nucleus anterior part to form acrosome cap or anterior nuclear cap (the cell is called early spermatid). In the acrosome cap center the acrosome (large lysosome) is formed (the cell is called late spermatid). The acrosome contains enzymes (hyaluronidase, trypsin, and other enzymes) taking part in the fertilization.
The proximal centriole is applied to the nucleus distal end while the distal centriole is divided into proximal and distal ringes. From the proximal ring (base body) the flagellum outgrows. The distal ring moves towards the end to form the boundary between the intermediate region and principal region of the flagellum.
At the time of the spermiogenesis the large part of the cell cytoplasm sheds off and the thin cytoplasm layer covers the spermatozoon. The mitochondria travel into the flagellum intermediate region.
Thus, the spermiogenesis is the transformation of spermatids into spermatozoon i.e., the spermatozoon converts in the individual moving cell. Before this time the spermatogenic cells have connected with each other by cytoplasm processes.
The mature spermatozoon consists of the head, including nucleus, acrosome, and flagellum including 1) neck, localized between the proximal centriole and proximal flagella ring of the distal centriole, 2) intermediate region located between the proximal and distal ringes, 3) principal region begun from the distal ring of the distal centriole, and 4) terminal region. In the flagellum center there is axoneme consisting of 9 pairs of peripheral and 1 pair of central tubules.
The Spermatogenesis Duration
Period from the spermatogonia division to the spermatozoon formation lasts 60 days. After it the spermatozoon matures 15 days. Thus, the spermatogenesis lasts 75 days.
It is necessary to note that the spermatogenesis within the seminiferous convoluted tubules is wave-like i.e., in the one part of the tubule the dividing spermatogonia are seen, in the second part the primary and secondary spermatocytes and spermatogonia are seen, in the third part the spermatogonia and spermatids are seen, and in the fourth part the spermatogonia, spermatids, and spermatozoa are seen.
Lack of the food and vitamins provides the harmful influence the spermatogenesis. The ionizing radiation and high temperature of the environment make the particularly harmful influence the spermatogenesis. These factors provide death of the spermatogenic cells present in the adluminal compartment of the seminiferous convoluted tubules (spermatozoa, spermatid, and spermatocytes), which stick together to form large spheres floating in the tubule fluid. Only due to the keeping spermatogonia present in the abluminal compartment of the seminiferous convoluted tubules the spermatogenesis may be renewing.
The high temperature, inhibiting the spermatogenesis, is the human body temperature. Therefore testis of the boy has to migrate into the scrotum, where the temperature is lower (34-360) than that of the body. If the testis does not travel into the scrotum (it is called cryptorchidism), the male becomes infertile.
The Testis Endocrine Function
The testis produces the testosterone, factor inhibiting the follicle-stimulating hormone, and factor stimulating the spermatogenic cell division.
The interstitial endocrine cells (Leidigs cells), producing a testosterone, localize around the blood vessels, surrounding seminiferous convoluted tubules. These oval cells contain oval nuclei. Their oxyphil cytoplasm contains vacuoles (vesicles) in its peripheral part. In the cytoplasm there are Golgi complex, smooth ER, and mitochondria containing vesicular cristae. Interstitial cells contain incorporations of the proteins, lipids, and glycogen. These cells are derived from the mesenchyme.
The testosterone influences the last stages of the spermatogenesis. The inhibitor produced by light sustaining epithelial cells, inhibit the follicle-stimulating hormone releasing. The factor, produced by dark sustaining epithelial cells, stimulates division of the spermatogenic cells.
Regulation of the Testis Function
The hypophysis hormones (follicle-stimulating hormone, luteinizing hormone, and prolactin) regulate spermatogenesis and endocrine functions of the testis. The role of the prolactin in male is not known.
The follicle-stimulating hormone stimulates multiplication of spermatogenic cells and synthesis of the androgen binding protein.
The luteinizing hormone stimulates releasing of the testosterone from the interstitial endocrine cells. The testosterone stimulates the last stages of the spermatogenesis. The female sex hormones inhibit releasing of the testosterone.
Thus, the follicle-stimulating hormone and the luteinizing hormone regulate the spermatogenesis. However the follicle-stimulating hormone influence immediately the multiplication of the spermatogenic cells but the luteinizing hormone stimulates the testosterone secretion the latter stimulates the spermatogenesis.
The Testis Nerve Supply
Sensitive nerve fibers (dendrites of sensitive ganglion neurons) end on the testis with receptors. In addition the efferent nerve fibers (sympathetic and parasympathetic) end on the testis with neuromuscular and neurosecretory terminals. However the sympathetic and parasympathetic nerve fibers poorly influence the endocrine function and spermatogenesis of the testis. The major influence the testis functions the hypophysis hormones provide.
Age Dependent Changes of the Testis
Soon after birth seminiferous convoluted tubules are similar to cords devoid of the lumen. The cords include sustaining epithelial cells and spermatogonia. At the age of 7 of the boy in the cords lumens are seen. At the age of 8-9 some part of spermatogonia undergoes differentiation to form primary spermatocytes. In the boys of 10-15 years old the secondary spermatocytes and spermatids are seen in seminiferous convoluted tubules. At the same time interstitial endocrine cells produce the testosterone stimulating rapid development of the epididymis, defferent duct, ejaculatory duct, and prostate. The testis involution begins in male after 50 years old. But spermatogenesis may be prolonged up to 80 years old.
The Seminiferous Tracts
The initial part of the seminiferous tracts is the straight seminiferous tubules running into the rete testis canaliculi. From the rete testis efferent ducts issue to form the epididymis head. The efferent ducts run into the epididymis duct, which is greatly coiled on it to form the epididymis body and tail. The duct of the epididymis continues with the defferent duct consisting of the descending and ascending limbs. The ascending limb reaches the scrotum exit. Here the duct wall part extends to form the seminal vesicle (gland). The seminal vesicle continues with the ejaculatory duct running into the prostate region of the urethra.
The structure of seminiferous tracts is similar to each other. The wall of the seminiferous tracts consists of 3 tunics: 1) the mucosal tunic, 2) the muscular tunic, and 3) the adventitial tunic. Different epithelium lines various parts of the tracts.
The columnar simple
epithelium lines the wall of the straight seminiferous tubules.
The simple cuboidal
or squamous epithelium lines the rete testis tubules
The efferent duct wall lumen surface is lined by two types of epithelial cells: 1) columnar ciliated cells, which the apical surface bears non-motile cilia, and 2) cuboidal cells releasing mucous secretion with the apocrine type discharging. Due to the different height of the epithelial cells the tubules lumen is star shaped.
The tall columnar cells and short basal cells that do not reach the lumen line the epididymis duct. The lumen surface of each tall columnar cell bears non-motile cilia. The basal cells provide the regenerative function.
The Epididymis Duct Signification
It serves as reservoir for spermatozoa. In the duct spermatozoa mature and are covered with the glycocalyx. The epithelial cells of the duct release fluid, which liquefies the sperm.
The defferent ducts has well developed smooth muscle tunic consisting of 3 layers: 1) internal longitudinal layer, 2) middle circular layer, and 3) external longitudinal layer. Between the layers there are nerve ganglions and number nerve terminals.
The ejaculatory duct contains less developed smooth muscles in its muscular tunic than those of the defferent duct. The smooth muscle cells of the seminiferous tract bear receptors, which are able to perceive the hypothalamus hormone oxytocin probably providing the simultaneous contraction of the seminiferous musculature at the time of the ejaculation.
The Seminal Glands (Vesicles)
It is the defferent duct bulging wall consisting of 3 layers: 1) the mucosal tunic, 2) the muscular tunic, and 3) adventitial tunic.
The mucosal tunic contains numerous folds. The tunic consists of 2 laminae: 1) epithelial lamina and 2) proper mucosal lamina. The epithelial lamina is made up of mucous cells, releasing mucous secretion, containing the fructose. In the proper mucosal lamina, consisting of collagen loose connective tissue there are glands, releasing their secretion into the seminal vesicle lumen.
The muscular tunic is made up of 2 laminae consisting of the smooth muscle: 1) internal circular muscular lamina and 2) external longitudinal lamina.
The adventitial tunic consists of the collagen loose connective tissue. The seminal glands release abundant fructose their alkaline secretion liquefies the sperm.
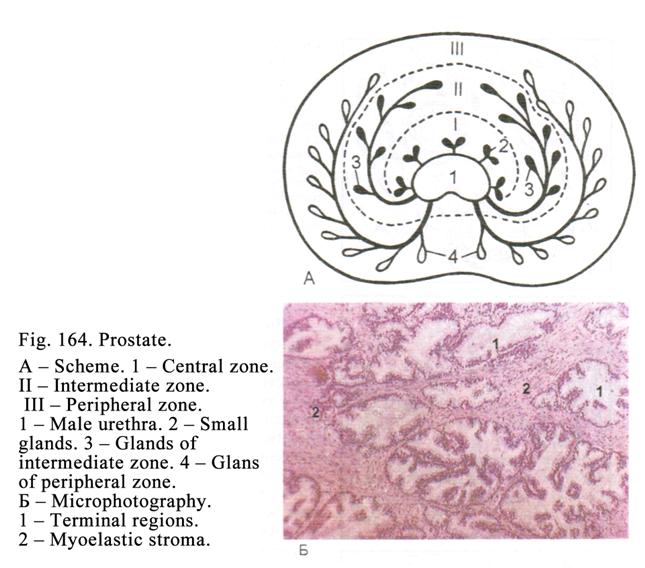
The Prostate
It surrounds the prostate part of the urethra.
The Prostate Development
The prostate
epithelium is derived from the urethral epithelium extending into surrounding mesenchyme.
Its connective tissue and smooth muscle are derived from the mesenchyme.
The Prostate Structure
The prostate (Fig. 164) consists of small glands surrounded by muscle and connective tissue (myoelastic stroma). The thin connective tissue capsule covers the prostate, from which septa issue, dividing the prostate into lobules
In the prostate there are 3 types of prostatic glands: 1) the smallest glands located in proper mucosal lamina of the urethra, 2) the intermediate glands located in the submucosal tunic of the urethra, and 3) the largest (peripheral) glands localized on the rest part of the prostate.
Every prostatic gland consists of the terminal region and the excretory duct. The mucous and basal cells, providing the regenerative function, line the terminal regions.
The pseudostratified columnar epithelium lines the excretory duct opening into the urethra. Around of the terminal regions and excretory ducts of prostate glands there are smooth muscle and connective tissue.
The seminal hillock bulges into the urethra lumen between the opening of the left and right ejaculatory ducts. The hillock consists of the collagen loose connective tissue containing number of the blood vessels and nerve endings (terminals). When the blood vessels have filled with blood, the seminal hillock becomes large and the urethra is closed. It prevents the sperm to pass into the urinary bladder at the time of the ejaculation.
Behind the seminal hillock the utriculus prostatic localizes. The utriculus is similar to the prostate gland. Its duct opens on the seminal hillock surface.
The prostate provides 1) producing the secretion, which liquefies the sperm, 2) production of the hormones (the growth factor and factor stimulating the testis function), and 3) participation in the differentiation of the hypothalamus to convert it from fetal hypothalamus into male hypothalamus at the time of the embryonic life.
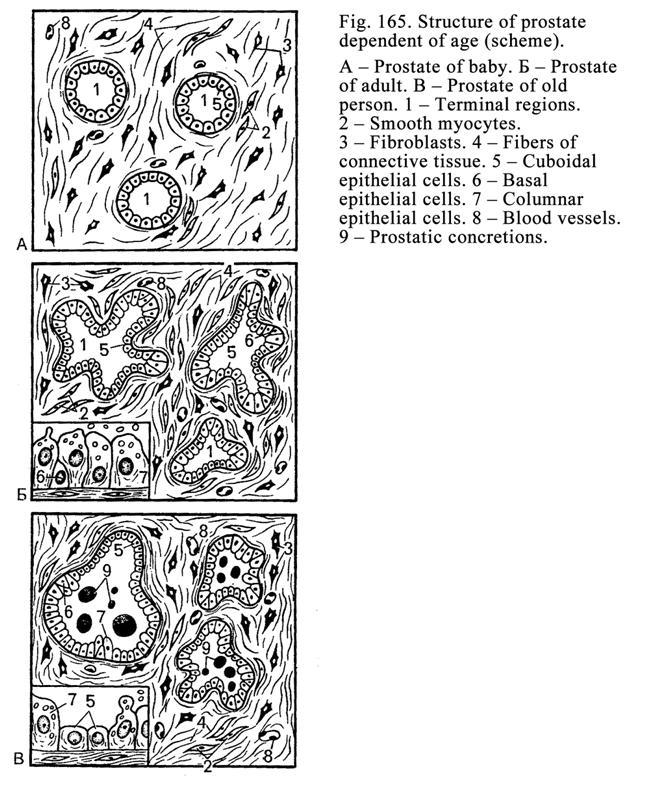
Age Dependent Changes of the Prostate
(Fig. 165)
Depending on the age the amount of the epithelium, connective tissue, and smooth muscle undergoes some changes. The simple cuboidal and columnar epithelium line the prostate tubular-alveolar gland terminal regions before puberty. In an adult (18-35 years old) the epithelium lining becomes only columnar and the amount of the smooth muscle increases. After 35 years old the prostate involution begins i.e., the epithelium lining becomes cuboidal, the amount of smooth muscle decreases while the connective tissue mass increase around the gland terminal regions and ducts.
In case of the low sexual activity the mucous secretion accumulates in the terminal regions of the prostate glands. At last the secretion becomes dense to form the prostate concretions. The increase of the connective tissue mass and formation of the prostate concretions lead to the growth of the prostate mass and the prostate urethra undergoes constriction and the passage of the urine stops. The doctor must prevent this disease.
Bulbourethral Glands
These are tubular-alveolar glands localized in the urethra upper part.
The bulbourethral gland ducts and terminal regions are irregular in shape. In some places their terminal regions, lined by mucous cells, fuse which each other to form dilated or narrowed alveoli. In the dilated alveoli the epithelium lining is flat but in the narrow alveoli it is cuboidal. Between the terminal regions there are the connective tissue and smooth muscle cells.
The bulbourethral glands release the mucous secretion into the urethra lumen.
The Penis
The penis consists of 2 dorsal corpora cavernosa and one corpus spongiosum (ventral corpus). The tunica albuginea, consisting of fibrous tissue, including collagen and elastic fibers, and smooth muscle cells, covers every corpus.
Between the corpora cavernosa there is collagen loose connective tissue in which blood vessels and nerve fibers are seen. In the corpus spongiosum (ventral corpus) the penile urethra traverses. The urethra includes 3 parts: 1) prostatic region, 2) membranous region, and 3) spongy region.
The Urethra Wall
It consists of 3 tunics: 1) mucosal tunic, 2) muscular tunic, and 3) adventitial tunic. The mucosal tunic of the prostatic region, passing through the prostate, is lined with transitional epithelium. The pseudostratified (columnar) epithelium, consisting of the columnar endocrine and goblet cells, lines the membranous region localized between the prostatic region and spongy region. The stratified squamous epithelium (partly keratinized) lines the spongy region passing through the corpus spongiosum. In the proper mucosal lamina there are mucous urethral glands releasing their secretion into the urethra.
The mucularl
tunic of the prostatic urethra consists of the
internal circular lamina and external longitudinal lamina. In the urethra
membranous region the muscular tunic is poorly developed. In the spongy region
there are single muscle bundles.
The adventitial tunic is made up of the collagen loose connective tissue.
The Glans Penis
The thin skin covers the glans. The glans penis is made up of the fibrous tissue containing a number of blood vessels and nerve endings.
The Penis Nerve Supply
Afferent and efferent (sympathetic and parasympathetic) nerve fibers and their terminals innervate the penis. The receptive apparatus includes the tactile and lamellar corpuscles.
The Penis Blood Supply
The penis artery is divided into helicine arteries localized in the connective tissue between the corpora cavernosa. In the usual condition of the penis these arteries have the helix appearance. In the internal tunic of the helicine artery there are bulges consisting of smooth muscle cells and collagen fibers. They are able to close the lumen of the arteries. In the artery middle tunic there is the abundant smooth muscle fibers.
From the helicine arteries the blood capillary vessels issue and run into cavernous spaces. The spaces, called lacunae, run into thin veins, running into the wide veins, having the thick walls, and containing abundant smooth muscle cells. When these vein wall smooth muscle cells contract, the thin veins are squeezed so the cavernous lacunae are filled with blood and the penis becomes large and hard.
CAPTER
27
FEMALE REPRODUCTIVE ORGANS
The female reproductive organs include ovaries, uterine tubes, uterus, vagina, and mammary glands.
The Female Genitalia Development
The epithelium and glands of uterine tubes and uterus and follicular epithelium are derived from mesoderm, the mature ovocytes are derived from the primordial germ cells those are formed in the yolk sac wall, and the connective and smooth muscle tissues are derived from mesenchyme.
The Ovary Structure
The peritoneum covers the ovary (Fig. 166). Deep to the peritoneum there is the tunica albuginea consisting of the fibrous tissue. Subjacent mass is the ovarian cortex. In the ovary center is the ovarian medulla, consisting of the connective tissue, containing convoluted veins and arteries. Here sometimes there are residues mesonephric tubules of the embryonic kidney those testify to the ovarian medulla are derived from the embryonic kidneys.
The Ovarian Cortex
It includes 1) ovarian follicles, 2) atretic follicles, 3) corpus luteum (exists 12-14 days every month), and 4) corpus albicans.
Ovarian Follicles
Depending on the development stage the ovarian follicles are divided into 1) primordial ovarian follicles, 2) primary ovarian follicles, 3) secondary ovarian follicles, and 4) tertiary ovarian follicles.
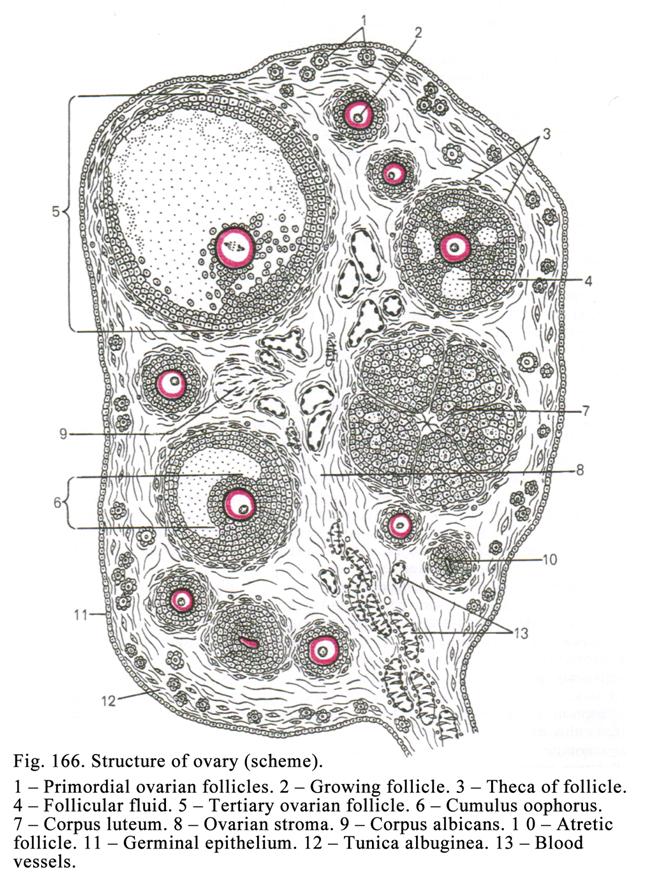
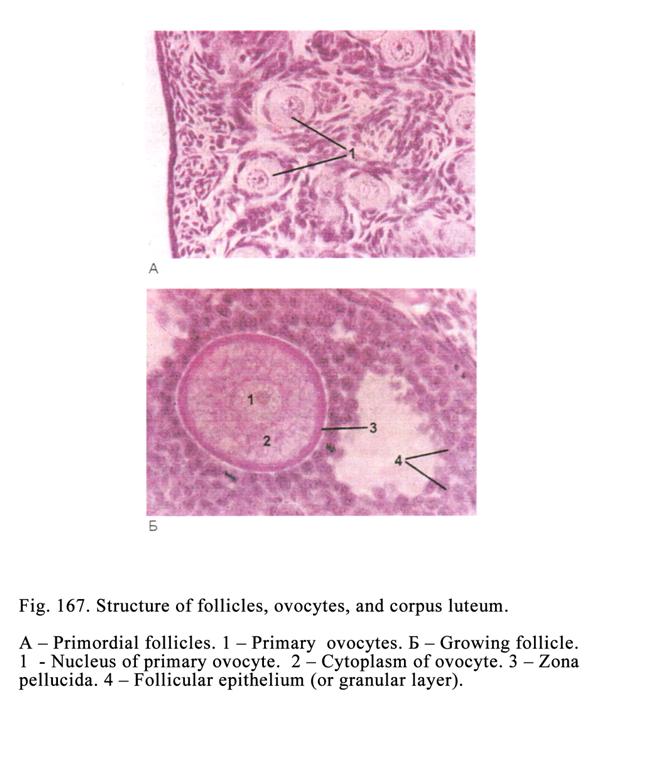
Primordial ovarian follicles (Fig. 166 1, Fig. 167 1) are the smallest and the most numerous follicles consisting of the primary ovocytes (on diplotene stage) surrounded by the layer of flattened follicular epithelial cells.
Primary ovarian follicles (Fig. 166 2) consist of growing primary ovocytes surrounded by one or two layers of cuboidal or columnar epithelial follicular cells. The basal ends of follicular cells rest on basal membrane. From the epical end of cells microvilli arise. The microvilli extend into the primary ovocyte cytoplasm. Through the microvilli the nourishing and others useful substances, providing the ovocyte growth, pass from follicular epithelial cells into the primary ovocyte. Round the primary ovocyte the second cell membrane, called the zona pellucida, is formed. The latter consists of glycosaminoglycans, proteoglycans, and proteins. The zona pellucida is formed due to the functional action of the ovocyte and follicular epithelial cells. In the follicular cells there is prominent synthetic apparatus, which synthesizes products necessary for the ovocyte growing and development. Due to the primary ovocyte growth and follicular epithelial cell proliferation the primary ovarian follicle becomes large. The follicle size increasing provides the surrounding connective tissue induration and the follicle capsule begins forming.
The secondary ovarian follicle (Fig. 166 6) is characterized by stoppage of the growth of the primary ovocyte. A number of folliccular epithelial cells locate round the primary ovocyte to form some layers of the cells forming the wide granular layer of the follicle. The follicle epithelial cells release liquor, which contains estrogen (female sex hormone). The liquor droplets accumulate to form the large drop and the cavity, called the follicular antrum, is formed. The antrum fluid quantity increases as a result the antrum becomes large. The part of the follicular cells, surrounding the ovocyte, along with the latter is pushed to another ovarian follicle pole to form the cumulus oophorus. The innermost layer of the follicular epithelial cells of cumulus oophorus and their processes, extending into ovocyte, form the third cell membrane of the ovocyte called the corona radiata.
Follicular cells of the secondary ovarian follicle provide the barrier function, trophic function, the follicular fluid formation, and the estrogen secretion.
Theca of the follicle is the follicle capsule consisting of the connective tissue. The theca of follicle includes the theca externa and theca interna. The theca externa is denser than the theca interna. In the theca interna a number of the blood vessels are seen. Around the blood vessels there are interstitial cells releasing the testosterone. The latter passes into granular layer of the follicle and is converted into the estrogen.
The secondary ovarian follicle rapidly grows due to the multiplication of the follicular epithelial cells and increasing of the follicular antrum.
The tertiary
ovarian follicles (Fig. 166 5)are characterized by
the large size und their further growing due to the proliferation of follicular
epithelial cells and the increase of the follicle antrum volume. Three
membranes surround the primary ovocyte of the tertiary follicle: 1)
plasmolemma, 2) zona pellucida, and 3) corona radiata. The follicle grows and
grows and it becomes 2-
After breaking of tertiary follicle the corpus luteum is formed instead of the tertiary follicle. After it the corpus luteum has destroyed and the corpus albicans is formed.
All secondary ovarian follicles (except one follicle) undergo degeneration (convert into atretic follicles).
The ovary provides 1) ovogenesis and 2) releasing of sex hormones.
The Ovogenesis
The ovogenesis includes 3 stages: 1) multiplication, 2) growth, and 3) maturation (the first and second meiotic division).
The multiplication
This stage starts and ends at the time of the embryonic life (the ovogonia have divided by mitosis).
The Growth Stage
It is divided into small growth and large growth. The small growth begins at the time of the embryonic life and ends by the puberty. At the time of the small growth the primary ovocyte on leptotene stage converts into ovocyte on the diplotene stage. After the puberty the primary ovocytes of the diplotene stage form the collection called the pile of ovocytes. The small growth stage does not need the activation with the follicle-stimulating hormone of the hypophysis. The small growth stage lasts 10-14 years.
After the puberty the follicle-stimulating hormone activates the large growth of the primary ovocytes. But not all ovocytes simultaneously begin growing (only 3-30 ovocytes). The large growth stage lasts 12-14 days. At the time of the large growth stage only one secondary follicle becomes the tertiary follicle. Within the tertiary follicle the primary ovocyte undergoes the first division.
The Maturation
At the time of the first meiotic division the primary ovocyte is divided into the secondary ovocyte, including almost entire cytoplasm, 23 dyads or 46 monads, three cell membrane (plasmolemma, zona pellucida, and corona radiata) and primary polar body including 23 dyads and small part of the cytoplasm. After it the tertiary follicle wall undergoes breaking and the secondary ovocyte is thrown out into the abdominal cavity and enter the uterine tube.
The second division occurs in the uterine tube after the secondary ovocyte fertilization. At the same time the ovocyte divides into the ovum, including 23 monads, almost entire cytoplasm, and 3 cell membranes and the secondary polar body containing 23 monads and cytoplasm small part.
Distinction of the Ovogenesis from the Spermatogenesis
At the time of the ovogenesis the multiplication stage starts and ends at the embryonic life while the spermatogenesis that occurs at the puberty. At the time of the ovogenesis the growth stage, including the small growth and large growth, starts at the embryonic life while at the spermatogenesis the growth stage is not divided into the small and large growth stages and occurs in seminiferous convoluted tubules of the adult. At the time of the ovogenesis the first division occurs within the tertiary ovarian follicle but the second division occurs within the uterine tube while at the spermatogenesis both the first and secondary divisions occur within the seminiferous convoluted tubules. The ovogenesis includes 3 stages (the spermiogenesis is absent) while the spermatogenesis includes 4 stages. At ovogenesis from one primary ovocyte one oocyte is only formed and three polar bodies at the time of the spermatogenesis from one primary spermatocyte four spermatids are formed.
The Ovulation
The ovulation is the throw of the secondary ovocyte from the tertiary follicle into the abdominal cavity. The hormonal changes in the female body are noted before the ovulation. Before 36 hours the ovulation the blood estrogen level becomes high. It leads to the follicle-stimulating hormone secretion inhibition. After it the intensive luteinizing hormone secretion starts from the hypophysis distal lobe. Before 12 hours of the ovulation the blood luteinizing hormone level becomes high (ovulation dose). At the same time in the tertiary follicle wall hyperemia occurs and the follicular fluid quantity increases so the intra-follicular pressure becomes high. The pressure influences the follicle wall therefore the latter undergoes the edema and infiltration by leucocytes so the wall becomes loose. At the same time the hyaluronidase activity becomes high, which splits the hyaluronic acid. It leads to the further loosing and weakness of the tertiary follicle wall and ovary tunica albuginea. The high intra-follicular pressure influences the receptors of the follicle wall to provide the nerve impulses reaching the hypothalamus. The impulses provide the oxytocin secretion, which takes part in the ovulation. All above noted factors provide breaking off the tertiary ovarian follicle wall and the tunica albuginea so the secondary ovocyte is thrown into the abdominal cavity.
The Corpus Luteum
After the ovulation the luteinizing hormone and prolactin influence the breaking follicle and instead of the follicle the corpus luteum develops. The corpus luteum development includes 4 stages: 1) the vascularization and proliferation stage, 2) the glandular metamorphose stage, 3) the active stage, and 4) the corpus luteum involution stage.
The Vascularization and Proliferation
Stage
It is the bleeding from the follicle wall broken blood vessels to form the hemorrhagic corpus, which is rapidly replaced by connective tissue scar. The follicular epithelial cells of the broken follicle undergo proliferation. The spaces between the proliferating cells blood vessels occupy (vascularization).
The Glandular Metamorphose Stage
The follicular
epithelial cells become large and undergo the differentiation and convert into
endocrine luteal cells.
The Active Secreting Stage
At this stage the corpus luteum is formed and the hormone progesterone is released. If the oocyte is not fertilized, the corpus luteum is called the corpus luteum of menstruation. If the oocyte is fertilized and pregnancy occurs, the corpus luteum is called the corpus luteum of pregnancy.
The corpus
luteum of menstruation persists for 12-14 days it
is 1.5-
The corpus
luteum of pregnancy persists for 3-4 months it is
5-
The Corpus Luteum Involution Stage
After the third stage the endocrine luteal cells undergo atrophy. Only the connective tissue scar persists. It converts into the corpus albicans.
The Atresia
All secondary ovarian follicles (except one) undergo atrophy to form the atretic follicles. One the secondary follicle reaches maturity, sheds an ovocyte, and becomes the corpus luteum. The atresia is the death of the ovocyte, its zona pellucida shrinks, and follicular cells disappear. At the same time the interstitial sells of the theca interna proliferate. These cells become large and have the appearance similar to that of the endocrine luteal cells. The atretic follicle differs from the corpus luteum because the former contains the shrinking zona pellucida of the ovocyte.
The Atresia Significations
The atresia significations are as follows: 1) the atretic follicles provide the hormonal function i.e., interstitial cells release the testosterone converting into the estrogen, 2) the atresia prevent the penetration of two or more spermatozoa into the oocyte, and 3) the atresia provides the natural (genetic) select within the organism. We have seen that several follicles (ovocytes) begin growing. But the best ovocyte grows rapidly than the rest ovocytes therefore the former outruns all others ovocytes and reach the maturation. Thereafter its follicular epithelial cells release inhibitory hormone, which provides the degeneration and atresia of the rest growing weakly ovocytes.
The Ovary Endocrine Function
The ovary produces 3 hormones: 1) progesterone, 2) estrogen, and 3) inhibitory hormone providing the degeneration and atresia of the weakly growing ovocytes (follicles). In additional the theca endocrine cells of the growing follicles and atretic follicles release the testosterone converting into estrogen.
The progesterone, produced by the corpus luteum, inhibits the large growth of the ovocytes. When the corpus luteum exists (12-14 days), the follicles cannot grow to enter the large growth stage. In addition the progesterone prepares the endometrium to the reception of the fertilized oocyte.
Three estrogens exist: estradiol, estrone, and estriol. The estrogens provide the restoration of the stratum functional of endometrium layer rejected after the menstruation.
The inhibitory hormone, produced by the follicular epithelial cells of secondary and tertiary follicles, provides the weakly growing follicles atresia.
The Endocrine Regulation of the Ovary
Function
The hypophysis anterior lobe hormones regulate the ovogenesis and hormonal function of the ovary. The luteinizing hormone provides the ovulation and function of the corpus luteum. The prolactin takes part in the formation and function of the corpus luteum and formation and function of breasts. The follicle-stimulating hormone influences the large growth of ovocytes and secretion of the estrogens from the growing follicles.
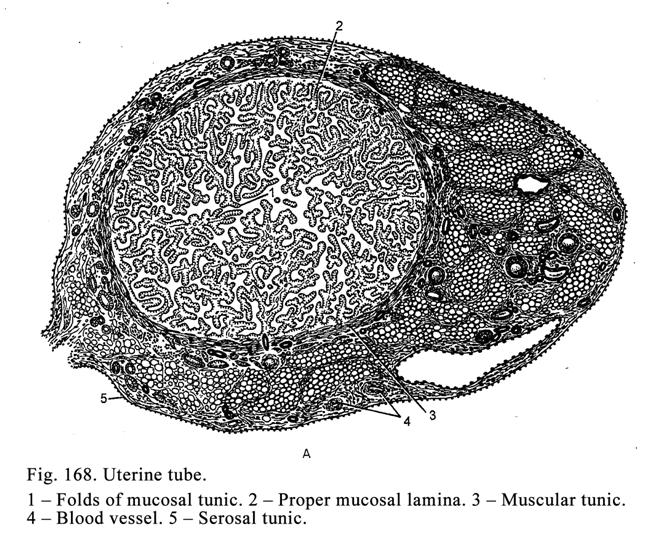
The releasing hormones of the hypothalamus stimulate the secretion of the follicle stimulating and luteinizing hormones from the hypophysis.
The hypothalamus hormone oxytocin stimulates the contraction of the myometrium and the discharge of the milk from the breast.
Uterine Tubes
The uterine tubes issue from the uterine angles. The uterine tube wall includes 3 tunics: 1) mucosal tunic, 2) muscular tunic, and 3) serosal tunic (Fig. 168).
The mucosal tunic consists of 2 laminae: 1) columnar epithelium, including ciliated cells, moving the oocyte towards the uterus, and mucous glandular cells, releasing the mucous secretion, and 2) proper mucosal lamina consisting of collagen loose connective tissue. The mucosal tunic forms folds, extending into the uterine tube lumen, to form the complicate labyrinth through which the oocyte moves towards the uterus during 5 days.
The muscular tunic consists of the internal circular layer and external longitudinal layer. The simultaneous contraction of both internal and external layers provides the oocyte movement towards the uterine.
The serosal tunic consists of connective tissue covered by mesothelium. The lateral dilated end of the tube is funnel shaped and is called the infundibulum. It is prolonged into a number of finger-like processes called fimbria. The core of the fimbria is the connective tissue, in which there are blood vessels. Before the ovulation and at the time of ovulation the fimbria become long and cover the ovary so the ovocyte, shedding off from ovary, enters the uterine tube, where undergoes the fertilization.
The Uterus
The uterus is pear shaped. It is a hollow organ consisting of the uterine body and uterine cervix. The uterus wall includes 3 tunics: 1) mucosal tunic (endometrium), 2) muscular tunic (myometrium), and 3) serosal tunic (Fig. 169).
The endometrium consists of column epithelium, including non-ciliated and ciliated epithelial cells, and proper mucosal lamina. The non-ciliated epithelial cells extend into the proper mucosal lamina to form uterine glands.
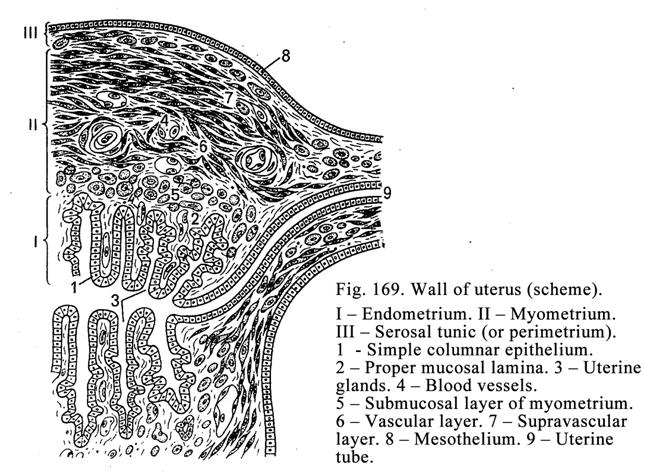
Functionally the endometrium is divided into 1) stratum functional of endometrium layer and 2) basal layer of endometrium.
The stratum functional of endometrium layer sheds off from the basal layer of endometrium and leaves the uterus along with the menstrual blood.
The basal layer of endometrium is constant. Due to this layer the stratum functional of the endometrium layer is restored after shedding off.
The myometrium consists of 3 layers: 1) submucosal layer, 2) vascular layer, and 3) supravascular layer.
The submucosal layer consists of the oblige smooth muscle cells.
The vascular layer is made up of the circular smooth muscle cells. In this layer there are the convoluted arteries, forming the arterial plexus, and convoluted veins forming the venous plexus.
The supravascular layer consists of the oblige smooth muscle cells directing perpendicularly in relation to that of the submucosal layer.
The smooth muscle cells of the uterus without the pregnancy are 20 mm in length in uterus at time of the pregnancy those are about 500 mm in length.
The serosal tunic (endometrium) consists of connective tissue covered by the mesothelium.
The parametrium (similar to endometrium), including a thick layer of adipose tissue, covers the anterior part and side aspects of the uterine cervix.
The Uterine Cervix
This is cylinder shaped. The cervix mucous tunic (endocervix) part, bulging into the vagina (vaginal portion of the cervix), is covered by the stratified squamous keratinized epithelium. The mucosal tunic, consisting of 2 layers (layer of the mucous columnar cells, releasing the mucus, and proper mucosal lamina including mucosal glands called the cervical uterine glands) lines the uterine cervix lumen.
The muscular tunic of the uterine cervix is made up of thick circular muscle cell layer, which serves, as might sphincter. Contraction of the sphincter stimulates the secretion from cervical uterine glands. The mucosal secretion as the cork closes the uterine cervix canal. When the sphincter relaxes, the uterine cervix lumen dilates to suck the sperm.
The Uterus Blood Supply
The uterine arteries enter the myometrium vascular layer to form the arterial plexus, from which small branches issue to all tunics and layers of the uterine wall.
In basal layer of the endometrium arterioles are straight while in the stratum functional of endometrium layer they assume the convoluted appearance. The arterioles branch into the capillary vessels, running into the venules, which run into the small veins, from those blood flows into the veins of uterine myometrium vascular layer.
The Uterus Nerve Supply
In the uterine wall there are a number of receptors, which are endings of the sensitive neuron dendrites.
To the uterus the
efferent nerve fibers (predominantly sympathetic) pass ending with nerve
terminals. Actions of different factors on the uterine receptors influence all
functional system of the female (cardio-vascular, respiratory, endocrine,
etc.).
The Vagina
The vagina wall (Fig. 170) consists of 3 tunics: 1) mucosal tunic, 2) muscular tunic, and 3) adventitial tunic.
The mucosal tunic is made up of the stratified squamous (cornified or non-cornified) epithelium and proper mucosal lamina. The epithelium includes 3 layers: 1) basal layer, 2) intermediate layer, and 3) superficial layer containing keratohyaline and glycogen granules. The glycogen undergoes splitting to form the acid medium destroying bacteria in the vagina.
The muscular tunic is made up of the longitudinal smooth muscle cell fascicles and single circular smooth muscle cells.
The adventitial tunic consists of the fibrous tissue.
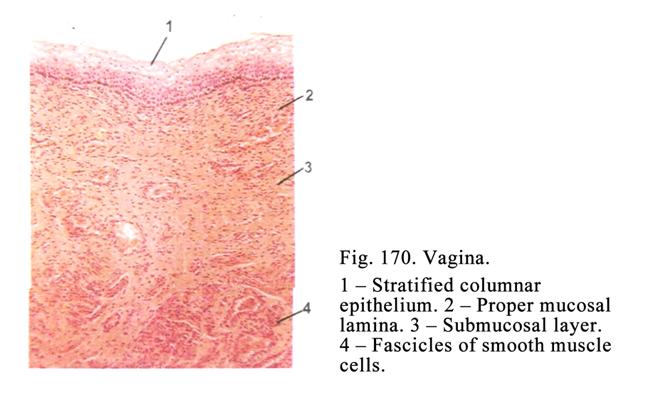
The Sexual Cycle
The female sexual system undergoes marked cyclical changes. Depending on the cyclical phase of the female there are changes in the hypothalamus, hypophysis, endometrium, mucosal tunic of the vagina, mucosal tunic of uterine tubes, and breasts. Cyclical change of endometrium is called the menstrual cycle.
The Menstrual Cycle
Normally the menstrual cycle (Fig. 171) lasts 28 days. The cycle includes 3 phases: 1) menstrual phase, 2) postmenstrual phase, and 3) premenstrual phase.
Before the menstrual phase (172 III) there happen the following changes. In the female hypothalamus the releasing hormone, which is concerned with the discharge of the luteinizing hormone, secretion is stopped and in the hypophysis the luteinizing hormone is also stopped. Thereafter the corpus luteum undergoes an involution it leads to the stoppage of the progesterone secretion so the convoluted arterioles of endometrium constrict. Therefore the blood supply of the stratum functional of endometrium layer is disturbed so the wall of convoluted arteries becomes weak. After the convoluted arterioles relax their wall undergoes rapture and the bleeding begins. The menstrual blood occupies the space between the basal layer endometrium and its functional stratum so the latter sheds off and flows out along with the menstrual blood. It is the beginning of the menstrual phase.
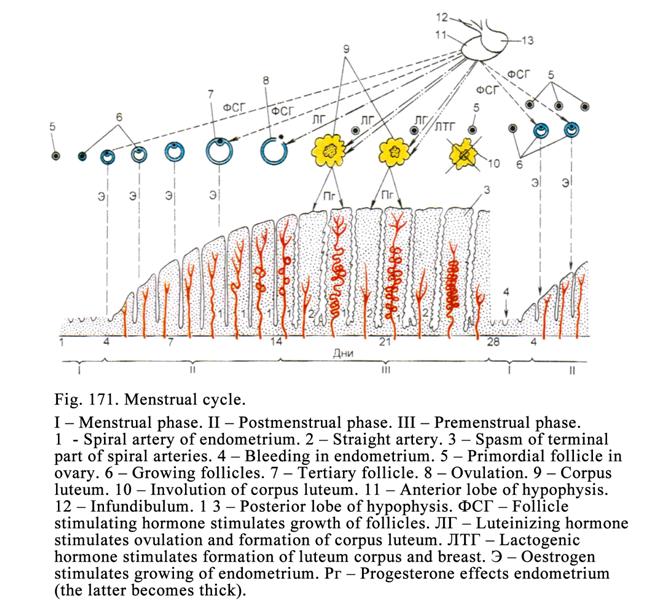
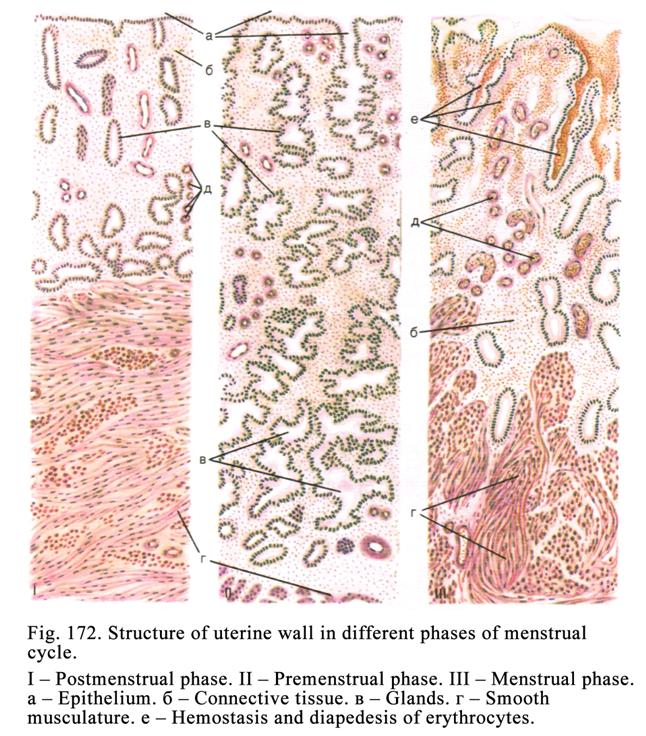
Normally the menstrual phase lasts 2 days. At the time of the menstrual phase the female sex hormone level is the lowest because the corpus luteum undergoes involution therefore the progesterone releasing stops. At the same time the ovarian follicles do not grow (the growth of the follicles is inhibited by the progesterone), so estrogens are not discharged.
At the time of the postmenstrual phase (Fig. 172 I) the releasing hormone discharges from the hypothalamus. This hormone influences the hypophysis, which releases the follicle-stimulating hormone providing the large growth of ovarian follicles. The growing follicles release the estrogen providing the restoration of the shedding off stratum functional of endometrium layer and uterine glands.
The postmenstrual phase lasts 12-14 days (until the ovulation). The restoration of the stratum functional of endometrium layer is particular during 5-11 days. At the same time the uterine glands are fully restored. They are straight but they do not contain any secretion. The stratum functional of the endometrium layer is also fully restored.
After the 11th day the period of the relative rest begins. During 12-14 days the anterior lobe of the hypophysis intensively releases the luteinizing hormone providing the ovulation.
After the ovulation the premenstrual phase (Fig. 172 II) begins. At the same time the luteinizing hormone influences the rapture follicle so the corpus luteum develops. The corpus luteum releases the progesterone influencing the premenstrual phase. At the time of this phase the endometrium undergoes edema and becomes thick, the uterine glands, filled with thick mucous secretion and containing much glycogen, assume the convoluted appearance. The mucosal tunic of the uterus prepares to receive the fertilizing oocyte.
If the oocyte has fertilized, the stratum functional of the endometrium layer converts in placenta maternal part, which sheds off from the uterine wall in 40 weeks (at the same time the birth occurs) and removes out of the uterus after the childbirth. If the oocyte has not fertilized, the stratum functional of endometrium layer sheds off from the uterus wall in 28 days after the menstrual phase and removes out of the uterus along with the menstrual blood and oocyte (birth the oocyte).
Vagina Mucosal Tunic Cyclic Changes
These changes are easy to be examined by smears receiving from vagina at the time of various menstrual phases. In the smear, received at the time of menstrual phase, the number erythrocytes and leucocytes are seen. In the smear, received at the time of postmenstrual phase, the quantity of erythrocytes and leucocytes sharply decreases, but the epithelial cells, containing pyknotic nuclei, are seen. At the end of the postmenstrual phase the quantity of the epithelial cells, containing pyknotic nuclei, increases blood cells are absent. At the initial of the premenstrual phase the quantity of the cells, containing pyknotic nuclei, is essential. At the midway of the premenstrual phase the quantity of cells, containing pyknotic nuclei, decreases but shedding off cells of the epithelium middle layer are seen. Before the menstrual phase the quantity of erythrocytes and leucocytes again increases.
The Vulva
The vulva, represented by vestibule of vagina, includes labia minora, labia majora, and clitoris. The vestibular glands open into vestibule of the vagina. The columnar epithelium lines the gland terminal regions. The glands release the mucous secretion. The stratum squamous keratinized epithelium, containing pigment granules, lines the labia minora. The proper mucosal lamina of the labia minora consists of the collagen loose connective tissue.
The labia majora are the skin folds predominantly consisting of the adipose tissue. In the labia there are sebaceous and sweat glands.
The clitoris is the rudimentary organ similar to penis, consisting of 2 highly sensitivity corpora cavernosa, ending glans, covered with the stratified squamous slightly keratinized epithelium.
The Vulva Nerve Supply
In vulva (particularly in clitoris) there are a number of nerve terminals. In vulva epithelium free endings are seen. In the proper mucosal lamina there are tactile corpuscles, in the dermis genital corpuscles are present, and in the labia majora there are lamillar corpuscles.
Age Dependent Changes of the Female
Genitalia
The newborn girl
uterus is about
The ovaries increase at early years due to the growth of their medulla. In the childhood the atresia of follicles is noted. Instead of these follicles the connective tissue grows. In the female before 30 years old the mass of the connective tissue also increases. At the climacterium period luteinizing hormone releasing decreases so the ovulation stops therefore the corpus luteum is not formed. Without the corpus luteum the progesterone is not produced. It leads to the menopause. Many ovarian follicles undergo atresia so the number of atretic follicles releases much estrogen. Therefore at the time of menopause the level of estrogens is high (during a short period).
The vagina of an old female undergoes atrophy, its lumen shrinks, mucosal tunic glands disappear, the vaginal mucus quantity decreases, the epithelium becomes thin, and in the epithelium the glycogen is absent therefore the vaginal medium becomes alkaline so the bacteria multiply and senile vaginits begins.
Mammary Glands
Although the mammary glands are present in both sexes they remain rudimentary in the male. In the female they are well developed after puberty (particularly after pregnancy).
The Mammary Gland Development
At the embryonic life on the body ventral skin surface 2 longitudinal cords from epidermis are formed. From every cord into subjacent mesenchyme some epithelial outgrowths extend. All these outgrowths except pectoral ones undergo atrophy. The pectoral outgrowths branch to form lactiferous ducts and alveolar ducts of mammary gland. In this condition the mammary glands are kept up to the pregnancy. At the time of the pregnancy on the alveolar ducts of mammary glands terminal regions (alveoli) are formed.
The Mammary Gland Structure before
Pregnancy
The mammary gland (breast) consists of 18-20 lobules separated from each other by the connective tissue septa. In every such lobule the compound
tubular-alveolar gland is present. The lactiferous duct of every gland opens on the nipple surface i.e., on nipple surface open 18-20 lactiferous ducts. The nipple is made up of thickened pigmented dermis. Before the lactiferous ducts open on the nipple surface they dilate to form lactiferous sinuses. Into these sinuses intralobular lactiferous ducts run. Into the latter the alveolar ducts run.
Depending on the menstrual cycle phase the mammary gland undergoes some changes. Before the menstrual phase on the alveolar duct surface of the mammary gland the alveoli are developed therefore the breast becomes large and dense. At the time of the menstrual phase these alveoli undergo involution so the mass and density of the breast decrease.
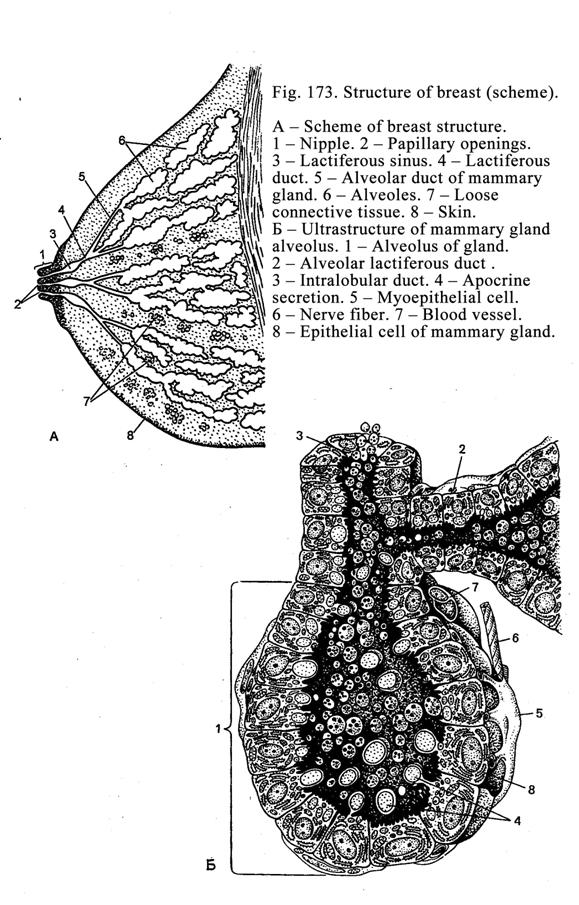
The
Lactiferous Mammary Gland Structure
The mammary gland fully develops at the time of the pregnancy. At the time of the pregnancy midway on the alveolar ducts of the mammary gland alveoli (Fig. 173) appear, in which colostrums accumulate. At the pregnancy end the lactiferous sinuses fill with colostrums. After the birth the colostrums are removed from the breast then epithelial cells of mammary gland alveoli synthesize the breast milk. Such breast is called the lactiferous mammary gland.
At the same time on alveolar ducts of mammary gland a number of alveoli, consisting of epithelial cells of mammary gland and stellate myoepithelial cells, is present. The stellate myoepithelial cells are located between the basal membrane and epithelial cells of mammary gland. The epithelial cells of the mammary gland are column shaped sometimes they are cone shaped. The apical surface of the cells bears microvilli. Desmosomes and adhesive belts connect these cells with each other. Their cytoplasm contains smooth ER, rough ER, mitochondria, Golgi complex, microfilaments, microtubules, and enzymes taking part in the milk synthesis. The
microtubules run from cell center towards its apical surface. Through the microtubules the lipid droplets move towards the cell apical surface and accumulate within the microvilli so the size of microvilli becomes large. After it the microvilli are separated from the cell and included into the breast milk (apocrine type of the secretion). At the same time these cells synthesize carbohydrates, proteins (globulins, albumins). The carbohydrates and proteins are released from the cells by exocytosis. The epithelial cells of the mammary gland release vitamins, antibodies, salts, and water. All these components, included into the breast milk, are the best useful substances for the breastfed infant.
The Breast Function Regulation
The nerve and endocrine systems regulate the breast function.
The Endocrine Regulation
The prolactin regulates the breast milk synthesis. The releasing hormone of the hypothalamus stimulates the prolactin synthesis in the hypophysis. The dopamine, discharged by the hypothalamus, inhibits the prolactin synthesis. When the prolactin is absent, the breast milk synthesis is stopped. The milk releasing from the breast is stimulated by the oxytocin of the hypothalamus. The oxytocin provides the contraction of the stellate myoepithelial cells stimulating the breast milk discharge from the epithelial cells of the mammary gland.
The Nervous Regulation
The nervous regulation is provided by the reflex arch. In the nipple there are a number of receptors (they are endings of the spinal ganglion sensitive neuron dendrites). From the receptors the impulse travels to the sensitive neuron. Through the
axon of the sensitive neuron the impulse is transmitted to neuron of the spinal cord nucleus. From here the impulse travels to the neuron of the sympathetic ganglion. The latter neuron axon transmits the impulse to stellate myoepithelial cells of the breast. The stellate myoepithelial cells contract and provide the discharge of the milk from the epithelial cells of the mammary gland.
The Differentiation of the Fetus
Hypothalamus Type into the Male Hypothalamus Type
The male hypothalamus differs from that of the fetus and of the female. The male hypothalamus includes one sex center while that of the fetus or the female includes 2 centers (low center and high ovulation center). The high center periodically stimulates the low center so the latter stimulates the hypophysis to release the ovulation dose of the luteinizing hormone providing the ovulation and development of the corpus luteum i.e., the sexual cycle begins.
The male hypothalamus sex (low) center constantly (every time) stimulates the anterior lobe of the hypophysis to release of luteinizing (interstitial cell stimulating hormone), providing the systematic secretion of the testosterone, and follicle stimulating hormone providing the spermatogenic cell division. Due to such structure of the male hypothalamus in male sex system the sex cycle, similar to menstrual cycle, is absent.
For the fetus hypothalamus to undergo differentiation from the female type into the male type it is necessary to inhibit the high center with a large quantity of the testosterone. For this purpose at the end of embryonic life the testis releases sufficiently large quantity of the testosterone. If the high sex center of the hypothalamus of the male fetus is not inhibited, the hermaphrodite is born.
Ąāņīš ļåšåāīäą Ļóćą÷åā Ģ. Ź.
Ļåšåāīä ńīīņāåņńņāóåņ šóńńźīé āåšńčč ėåźöčé Źóēķåöīāą Ń. Ė. č Ļóćą÷åāą Ģ. Ź.,
«Ėåźöčč ļī ćčńņīėīćčč, öčņīėīćčč č żģįščīėīćčč»
(3-å čēäąķčå), čēäąņåėüńņāī ĢČĄ, Ģīńźāą, 2014).